Articles from ViiV Healthcare

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders,* today announced positive 12-month interim efficacy and tolerability data from the phase IIb EMBRACE study.1 In adults living with HIV on stable therapy, a regimen of lotivibart (N6LS), a broadly neutralising antibody, administered every four months combined with monthly intramuscular (IM) long-acting cabotegravir (CAB LA), maintained viral suppression in 94% of participants receiving lotivibart intravenously (IV) and 82% subcutaneously (SC), compared with 88% in the standard of care group.
By ViiV Healthcare · Via Business Wire · February 25, 2026

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders,* today announced data from its phase 1 study of long-acting formulations of VH184, the first, third-generation integrase strand transfer inhibitor (INSTI) in development for HIV.1 Results show a single-dose injection could maintain drug levels for up to six months. In-vitro data from a separate study demonstrate improved potency and an enhanced resistance profile versus bictegravir in resistant HIV strains.2
By ViiV Healthcare · Via Business Wire · February 25, 2026

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders,* today announced final data from the LATITUDE phase III trial, confirming its long-acting injectable treatment for HIV, Cabenuva (cabotegravir + rilpivirine), demonstrated superior efficacy in maintaining viral load suppression compared to daily oral therapy in individuals with a history of antiretroviral treatment (ART) adherence challenges.1
By ViiV Healthcare · Via Business Wire · February 18, 2026

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders,* today announced data presentations from its innovative HIV treatment and prevention portfolio and integrase inhibitor (INSTI)-led pipeline at the 33rd Conference on Retroviruses and Opportunistic Infections (CROI) in Denver, Colorado from 22-25 February.
By ViiV Healthcare · Via Business Wire · February 17, 2026

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced 96-week findings from PASO DOBLE (GeSIDA 11720 study) showing that Dovato (dolutegravir/lamivudine [DTG/3TC]) is as effective as Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide fumarate [BIC/FTC/TAF]) in maintaining virological suppression in adults with HIV-1. Individuals taking Dovato also experienced statistically significant less weight gain and fewer drug-related adverse events over the two-year study period. Results will be presented at the European AIDS Clinical Society (EACS) annual congress, held in Paris, France (15-18 October).
By ViiV Healthcare · Via Business Wire · October 17, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced data from the phase I CLARITY open-label, crossover study, showing clinically relevant differences in injection site reaction (ISR) acceptability and tolerability, with 69% of HIV-negative adults finding cabotegravir long-acting (CAB LA) injections to be “totally or very acceptable” vs 48% for lenacapavir (LEN) injections. Data presented at the 20th European AIDS Conference (EACS) in Paris, France, also showed 90% of HIV-negative adults and 86% of healthcare providers (HCPs) preferred CAB LA injections over LEN injections after a single dose, and ISR events were more frequent and visible with LEN than with CAB LA.1
By ViiV Healthcare · Via Business Wire · October 15, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced the presentation of more than 60 abstracts, including data from its industry-leading long-acting HIV treatment and prevention portfolio, at the 20th European AIDS Conference (EACS) in Paris, France from 15-18 October, and at IDWeek 2025 in Atlanta, Georgia, US from 19-22 October.
By ViiV Healthcare · Via Business Wire · October 9, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced data from the phase IIIb VOLITION study demonstrating that 89% (n=129/145) of eligible treatment-naïve people living with HIV opted to switch to long-acting injectable Vocabria + Rekambys (branded as Cabenuva in the US Canada and Australia) following rapid viral suppression with daily Dovato (dolutegravir/lamivudine (DTG/3TC)). Additional real-world data from other studies reinforce CAB+RPV LA’s effectiveness across a broad range of populations.
By ViiV Healthcare · Via Business Wire · July 14, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced an update to their voluntary licensing agreement with the Medicines Patent Pool (MPP) for cabotegravir to include patents relating to its use in a long-acting HIV treatment regimen. The announcement follows updated guidance from WHO recommending long-acting injectable cabotegravir + rilpivirine as an HIV treatment option.
By ViiV Healthcare · Via Business Wire · July 14, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced the presentation of abstracts from its innovative HIV treatment and prevention portfolio at the International AIDS Society 2025 Conference (IAS 2025) in Kigali, Rwanda. Key data will highlight the long-term effectiveness of Vocabria & Rekambys, (branded as Cabenuva in the US, Canada and Australia) the company’s complete long-acting injectable regimen for treatment; evaluate patient preference for long-acting injectables compared to daily oral therapy; and measure benefits of long-acting injectables in tackling common challenges associated with taking daily pills, including stigma and adherence.
By ViiV Healthcare · Via Business Wire · July 8, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced new data from two implementation studies showing zero cases of HIV acquisition for Apretude, the only long-acting injectable approved for HIV prevention. Real-world data were also presented for Cabenuva, the only approved, complete long-acting injectable treatment regimen, showing its effectiveness in the three years since it has been available.
By ViiV Healthcare · Via Business Wire · March 12, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced positive findings from the company’s EMBRACE phase IIb study.1 The study found that N6LS (VH3810109 or VH109), given every four months in combination with monthly cabotegravir long-acting (CAB LA), successfully kept viral levels suppressed in adults living with HIV who were already stable on treatment. It was also well tolerated by participants.
By ViiV Healthcare · Via Business Wire · March 12, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced positive findings from two phase IIa proof-of-concept studies of investigational antiretroviral therapies VH4524184 (VH184) and VH4011499 (VH499). These findings, presented at the Conference on Retroviruses and Opportunistic Infections (CROI 2025) in San Francisco, U.S., support the continued development of these two compounds as distinct options for a new generation of long-acting injectables for HIV, with the potential for extended dosing intervals.
By ViiV Healthcare · Via Business Wire · March 11, 2025

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced 48-week findings from the DOLCE study, sponsored by Fundación Huésped and the Bahiana Foundation of Infectiology, showing the 2-drug regimen Dovato (dolutegravir/lamivudine [DTG/3TC]) achieved similar results to 3-drug therapy in viral suppression in a population of adults with advanced HIV. A post-hoc analysis of the study showed DTG/3TC was non-inferior to 3-drug therapy regardless of the participant’s baseline viral load. These data were presented at HIV Glasgow 2024, being held in Glasgow, Scotland from 10 – 13 November.
By ViiV Healthcare · Via Business Wire · November 11, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, will share new data from its industry-leading HIV treatment and prevention portfolio and pipeline at HIV Glasgow 2024, being held in Glasgow, Scotland from 10 – 13 November 2024. Spanning 42 abstracts, the data showcases long-acting and two-drug regimens as care options for diverse populations in the face of a continuing HIV epidemic.
By ViiV Healthcare · Via Business Wire · November 7, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced the presentation of new real-world evidence and implementation data showing the effectiveness, adherence, and quality-of-life improvement of Apretude (cabotegravir long-acting (CAB LA)) for HIV pre-exposure prophylaxis (PrEP). The data will be presented at IDWeek 2024, being held in Los Angeles, California from 16 – 19 October.
By ViiV Healthcare · Via Business Wire · October 16, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced the company’s commitment to make at least two million doses of long-acting cabotegravir for HIV pre-exposure prophylaxis (CAB LA for PrEP) available for procurement for low- and middle-income countries (L&MICs) during 2025-2026. The new commitment will triple the company’s available supply versus 2024, to accelerate access and meet growing demand where the HIV burden and unmet need are greatest. The announcement was made at the HIV R4P 2024 conference, being held in Lima, Peru from 6 – 10 October.
By ViiV Healthcare · Via Business Wire · October 7, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, announces 48-week findings from PASO DOBLE (GeSIDA 11720 study), the largest head-to-head, phase IV randomised clinical trial (RCT) investigating the 2-drug regimen Dovato (dolutegravir/lamivudine [DTG/3TC]) compared to the 3-drug regimen Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide fumarate [BIC/FTC/TAF]) for the treatment of HIV-1 in people who are virologically suppressed and who could benefit from treatment optimisation.1
By ViiV Healthcare · Via Business Wire · July 23, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced it will be presenting the largest head-to-head, randomised clinical trial (RCT) of the 2-drug regimen Dovato (dolutegravir/lamivudine [DTG/3TC]) compared against the 3-drug regimen, Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide [BIC/FTC/TAF]) at the 25th International AIDS Conference in Munich, Germany (22 – 26 July). The presentation is one of 25 abstracts evaluating the company’s portfolio of marketed HIV treatment and prevention options alongside its next-generation pipeline assets.
By ViiV Healthcare · Via Business Wire · July 15, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced the U.S. Food and Drug Administration (FDA) approved Dovato (dolutegravir/lamivudine) for the treatment of HIV-1 infection in adolescents 12 years of age and older and weighing at least 25 kg with no antiretroviral (ARV) treatment history or to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies/mL) on a stable ARV regimen with no history of treatment failure and no known substitutions associated with resistance to the individual components of Dovato.1
By ViiV Healthcare · Via Business Wire · April 8, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer Inc. and Shionogi Limited as shareholders, today announced data from a planned interim analysis of the LATITUDE phase III trial, indicating that their long-acting injectable antiretroviral treatment (ART) for HIV, Cabenuva (cabotegravir + rilpivirine), demonstrated superior efficacy in maintaining viral load suppression compared to daily oral therapy in individuals with a history of ART adherence challenges.
By ViiV Healthcare · Via Business Wire · March 6, 2024

Today, ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, and the American Academy of HIV Medicine (AAHIVM) jointly announced the launch of The Dr. Dawn K. Smith HIV Prevention Clinical Fellowship. The fellowship will provide comprehensive HIV training to non-infectious disease clinicians serving communities disproportionately affected by HIV. The fellowship was announced at the Conference on Retroviruses and Opportunistic Infections (CROI 2024), one of the world’s largest convenors of the HIV scientific and medical community, in Denver, Colorado.
By ViiV Healthcare · Via Business Wire · March 5, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced positive findings from its phase I study showing that an investigational formulation of cabotegravir, known as cabotegravir ultra long-acting (CAB-ULA), can be dosed at intervals of at least four months. This is the company’s first step towards delivering ultra long-acting injectable HIV treatment and prevention medicines that would potentially enable people to have at least four months between visits to the clinic.
By ViiV Healthcare · Via Business Wire · March 4, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced the presentation of 64 abstracts that includes highlights of the company’s next-generation pipeline advancements, alongside data from its diverse portfolio of marketed HIV treatment and prevention options at the Conference on Retroviruses and Opportunistic Infections (CROI 2024) being held in Denver, Colorado, from 3 – 6 March 2024.
By ViiV Healthcare · Via Business Wire · February 28, 2024
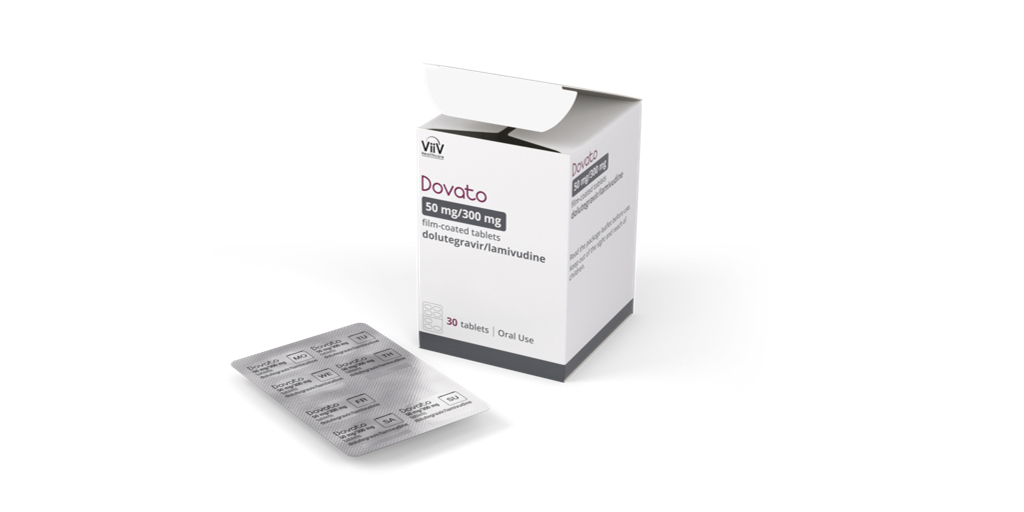
ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced Dovato (dolutegravir/ lamivudine) is now available in a blister pack in the U.S. Dovato is approved as a complete regimen to treat HIV-1 infection in adults with no antiretroviral (ARV) treatment history or to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable ARV regimen with no history of treatment failure and no known resistance to any component of Dovato.
By ViiV Healthcare · Via Business Wire · February 6, 2024

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced the presentation of key abstracts highlighting the breadth of its approved and investigational medicines at the 19th Annual European AIDS Conference (EACS 2023) being held in Warsaw, Poland from 18-21 October 2023.
By ViiV Healthcare · Via Business Wire · October 18, 2023

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced positive 12-month findings from the SOLAR study. SOLAR is the first head-to-head, Phase IIIb study of the first and only complete long-acting injectable regimen Cabenuva (cabotegravir, rilpivirine [CAB+RPV LA]) compared against complete daily oral regimen Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide [BIC/FTC/TAF]).
By ViiV Healthcare · Via Business Wire · February 23, 2023

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced the presentation of key abstracts from the company’s diverse portfolio of industry-leading innovative HIV treatment and prevention options alongside next-generation pipeline advancements at the Conference on Retroviruses and Opportunistic Infections (CROI 2023) being held in Seattle, Washington from 19 – 22 February 2023.
By ViiV Healthcare · Via Business Wire · February 14, 2023

Today on World AIDS Day, the Health Action Alliance and ViiV Healthcare announce the launch of U.S. Business Action to End HIV, a new coalition of businesses committed to accelerating progress to end HIV in the U.S. by 2030. Ada Health, Avita, BLK, Chispa, CVS Health, Gilead Sciences, Healthvana, National LGBT Chamber of Commerce, OraSure Technologies, The Powell Companies Real, Tinder, Uber, ViiV Healthcare, Walgreens and Walmart are founding member organizations of the coalition.
By ViiV Healthcare · Via Business Wire · December 1, 2022

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced new data from a perceptions survey which unveils a widespread gap in public knowledge and understanding of HIV worldwide. The data found that three quarters of people (74%) believe that there are still negative perceptions when it comes to people living with HIV, and that one in six adults agree that if a friend or colleague was living with HIV, they might look at them negatively. While the majority of people surveyed did report feeling comfortable with physical contact, including holding hands (76%) or hugging (75%), only 1 in 2 people would feel comfortable dating someone living with HIV. Concerningly, the survey also found that a quarter of adults (25%) believe that it is not appropriate for employees to talk about HIV in the workplace, a factor continuing to perpetuate HIV as a taboo subject.1
By ViiV Healthcare · Via Business Wire · November 30, 2022

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced positive findings from its phase IIa proof-of-concept study of N6LS (VH3810109), a novel, investigational, broadly neutralising antibody (bNAb) that is being studied at two dosing levels – a high dose and ten-fold lower dose (40 mg/kg and ~4 mg/kg (280 mg), respectively), in adults living with HIV. The study showed that a single infusion of N6LS demonstrated strong antiviral efficacy at both doses while also being well-tolerated by study participants.1 The results were presented today at the 30th HIV Glasgow Conference being held in Glasgow, Scotland from 23 – 26 October.
By ViiV Healthcare · Via Business Wire · October 25, 2022

ViiV Healthcare, the global specialist HIV company majority-owned by GSK, with Pfizer and Shionogi as shareholders, today presented findings from the CARISEL study (Cabotegravir And Rilpivirine Implementation Study in European Locations). The study, which evaluated the perspectives of people living with HIV and healthcare teams through surveys and interviews in addition to evaluating clinical effectiveness, demonstrated that ViiV Healthcare’s Vocabria (cabotegravir injection) and Janssen Pharmaceutical Companies of Johnson and Johnson’s Rekambys (rilpivirine long-acting injectable suspension) was successfully implemented across a range of European healthcare settings.1 The 12-month findings from the implementation study of the first and only complete long-acting regimen for HIV treatment were presented today at the 30th HIV Glasgow Conference being held in Glasgow, Scotland and virtually, from 23 – 26 October.
By ViiV Healthcare · Via Business Wire · October 24, 2022

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, today announced week 240 results from the phase III BRIGHTE study of fostemsavir in heavily treatment-experienced (HTE) adults with multidrug-resistant HIV-1 infection who have very few treatment options left available to them due to resistance, intolerance, or other safety concerns.
By ViiV Healthcare · Via Business Wire · July 29, 2022

ViiV Healthcare, the global specialist HIV company majority-owned by GSK, with Pfizer and Shionogi as shareholders, today announced new efficacy and safety findings from the unblinded period of the HIV Prevention Trials Network (HPTN) 084 trial evaluating cabotegravir long-acting (LA) for pre-exposure prophylaxis (PrEP) in women in sub-Saharan Africa. The findings showed that cabotegravir LA for PrEP continued to demonstrate superior efficacy in the prevention of new HIV infections among women when compared to daily oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) tablets, with an 89% lower rate of HIV acquisition (Hazard Ratio 0.11, 95% Confidence Interval [CI] 0.05, 0.24).1 Results were presented today at the 24th International AIDS Conference (AIDS 2022) in Montreal, Canada.
By ViiV Healthcare · Via Business Wire · July 28, 2022

ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, and the Medicines Patent Pool (MPP) today announced the signing of a new voluntary licensing agreement for patents relating to cabotegravir long-acting (LA) for HIV pre-exposure prophylaxis (PrEP) to help enable access in least developed, low-income, lower middle-income and Sub-Saharan African countries1,2.
By ViiV Healthcare · Via Business Wire · July 28, 2022

ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline plc (“GSK”), with Pfizer Inc. and Shionogi B.V. as shareholders, today announced that the US Food and Drug Administration (FDA) has approved Cabenuva (cabotegravir, rilpivirine) for the treatment of HIV-1 in virologically suppressed adolescents (HIV-1 RNA less than 50 copies per milliliter [c/mL]) who are 12 years of age or older and weigh at least 35kg on a stable antiretroviral regimen, with no history of treatment failure, and with no known or suspected resistance to either cabotegravir or rilpivirine.1, 2 The regimen was co-developed as part of a collaboration with the Janssen Pharmaceutical Companies of Johnson & Johnson. This approval marks the first time a long-acting HIV treatment is available for the adolescent population, underscoring ViiV Healthcare’s commitment to delivering options for young people living with HIV.
By ViiV Healthcare · Via Business Wire · March 29, 2022

For media and investors only
By ViiV Healthcare · Via Business Wire · March 24, 2022

ViiV Healthcare, the global specialist HIV company majority-owned by GlaxoSmithKline plc (GSK), with Pfizer Inc. (Pfizer) and Shionogi Limited (Shionogi) as shareholders, today announced the presentation of company and investigator sponsored abstracts from its industry-leading HIV treatment and prevention portfolio of approved long-acting medicines and 2-drug regimens (2DRs) at the Conference on Retroviruses and Opportunistic Infections (CROI 2022), being held virtually 12-16 February.
By ViiV Healthcare · Via Business Wire · February 7, 2022
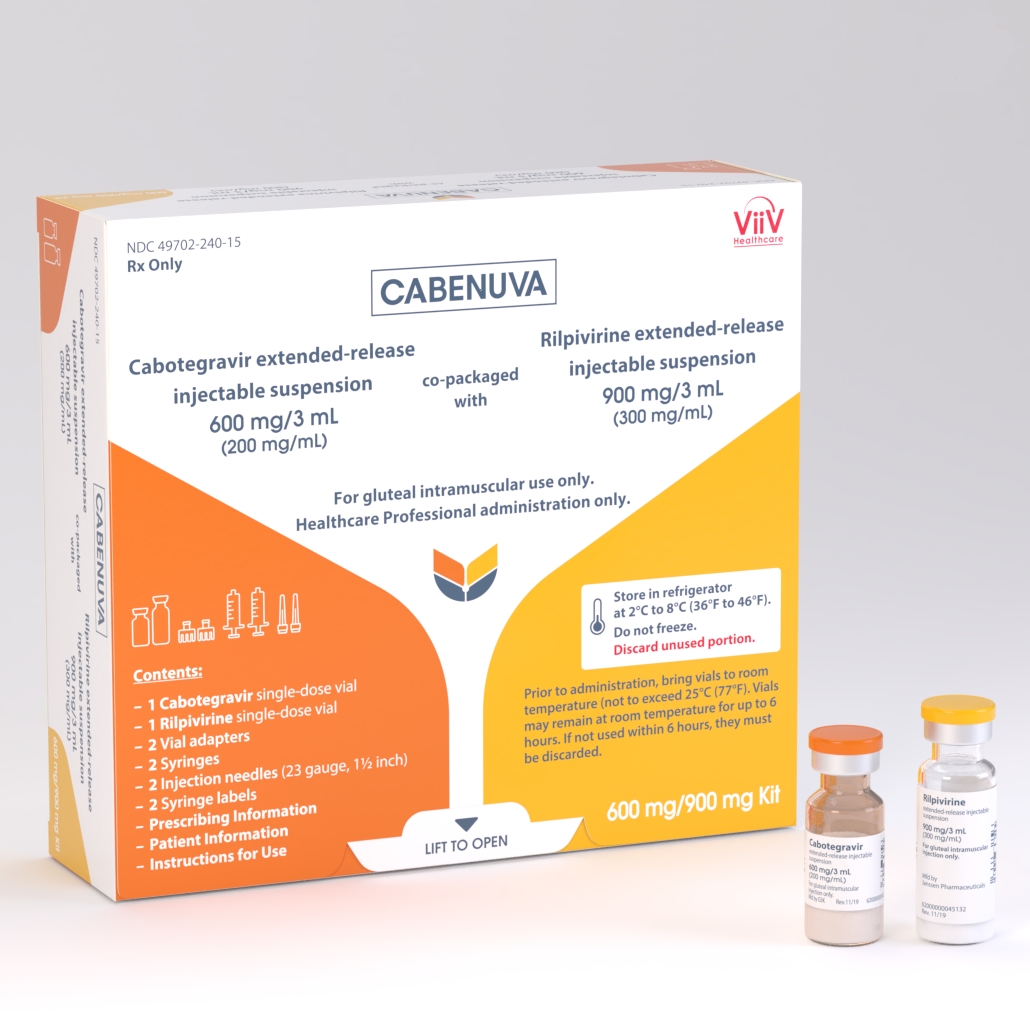
ViiV Healthcare, the global specialist HIV company majority-owned by GlaxoSmithKline plc (GSK), with Pfizer Inc. (Pfizer) and Shionogi Limited (Shionogi) as shareholders, today announced that the US Food and Drug Administration (FDA) approved Cabenuva (cabotegravir, rilpivirine) for every-two-month dosing for the treatment of HIV-1 in virologically suppressed adults (HIV-1 RNA less than 50 copies per millilitre [c/ml]) on a stable regimen, with no history of treatment failure, and with no known or suspected resistance to either cabotegravir or rilpivirine.
By ViiV Healthcare · Via Business Wire · February 1, 2022

ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline plc (GSK), with Pfizer Inc. (Pfizer) and Shionogi Limited (Shionogi) as shareholders, today announced that the US Food and Drug Administration (FDA) approved Apretude, the first and only long-acting injectable pre-exposure prophylaxis (PrEP) option to reduce the risk of sexually acquired HIV-1. The long-acting injectable was approved for use in adults and adolescents weighing at least 35 kg who are at risk of sexually acquiring HIV and who have a negative HIV-1 test prior to initiation. The medicine was studied in men who have sex with men, as well as women and transgender women who have sex with men, who were at increased risk of sexually acquiring HIV.
By ViiV Healthcare · Via Business Wire · December 20, 2021

Pervasive stigma and prejudice towards people living with HIV continues to persist, according to a new international survey released today on World AIDS Day. Funded by ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline Plc (“GSK”), with Pfizer Inc. and Shionogi Limited as shareholders, the survey uncovers outdated attitudes and inherent bias amongst the general public towards people living with HIV. An overwhelming majority (88%) of respondents believe there are still negative perceptions towards people living with HIV, even though it can now be effectively managed with antiretroviral (ARV) medication. Concerningly, almost a third (30%) of people surveyed incorrectly believe HIV can be transmitted through kissing.
By ViiV Healthcare · Via Business Wire · November 30, 2021

For media and investors only
By ViiV Healthcare · Via Business Wire · October 28, 2021

For media and investors only
By ViiV Healthcare · Via Business Wire · October 28, 2021

ViiV Healthcare today announced the release of Achieving health equity: a roadmap to eliminating disparities, a new report conducted by Economist Impact, a division of The Economist Group, that explores the opportunities that could be created by eliminating health disparities in the UK and US over the next 20 years, while charting a bold course for unified action. The report, sponsored by ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline plc (GSK), with Pfizer Inc. and Shionogi Limited as shareholders, breaks from previous efforts by laying out a roadmap that identifies the role of individual groups in achieving health equity and describes the concrete actions they can take to address disparities.
By ViiV Healthcare · Via Business Wire · October 20, 2021

For media and investors only
By ViiV Healthcare · Via Business Wire · September 29, 2021

ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline plc (GSK), with Pfizer Inc. and Shionogi as shareholders, today announced that the U.S. Food and Drug Administration (FDA) has accepted and granted Priority Review for a New Drug Application (NDA) for investigational, injectable cabotegravir long-acting for pre-exposure prophylaxis, or PrEP. The Priority Review designation of cabotegravir long-acting for PrEP builds upon its prior identification as a Breakthrough Therapy by the FDA.
By ViiV Healthcare · Via Business Wire · September 28, 2021

ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline (GSK), with Pfizer and Shionogi as shareholders, today announced an exclusive collaboration and license agreement with Shionogi & Co. Ltd. (Shionogi) for S-365598, a third-generation investigational integrase strand transfer inhibitor (INSTI) with potential for use in ultra long-acting HIV regimens (regimens with dosing intervals of three months or longer).
By ViiV Healthcare · Via Business Wire · September 28, 2021

For media and investors only
By ViiV Healthcare · Via Business Wire · July 17, 2021

For media and investors only
By ViiV Healthcare · Via Business Wire · July 17, 2021

ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline plc (“GSK”), with Pfizer Inc. and Shionogi Limited as shareholders, and Halozyme Therapeutics, Inc. (Nasdaq: HALO) today announced a global collaboration and license agreement that gives exclusive access to Halozyme’s ENHANZE® drug delivery technology, recombinant human hyaluronidase PH20 enzyme (rHuPH20), for specific targets used in the treatment and prevention of HIV.
By ViiV Healthcare · Via Business Wire · June 22, 2021

ViiV Healthcare, the global specialist HIV company majority owned by GlaxoSmithKline plc (“GSK”), with Pfizer Inc. and Shionogi Limited as shareholders, today announced the initiation of a rolling submission of a new drug application (NDA) with the US Food and Drug Administration (FDA) for investigational, long-acting, injectable cabotegravir for the prevention of HIV, also called pre-exposure prophylaxis, or PrEP.
By ViiV Healthcare · Via Business Wire · May 4, 2021
