Articles from Revelation Biosciences, Inc.

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, today reported its financial results for the three and six months ended June 30, 2025.
By Revelation Biosciences, Inc. · Via Business Wire · August 8, 2025

Revelation Biosciences, Inc. (Nasdaq: REVB) the “Company” or “Revelation”) a clinical-stage life sciences company focused on rebalancing inflammation to optimize health, today congratulates its former chairman and shareholder, George Tidmarsh, MD, PhD, who has been selected to head the FDA Center for Drug Evaluation and Research.
By Revelation Biosciences, Inc. · Via Business Wire · July 24, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company focused on rebalancing inflammation to optimize health, today announced dosing has been completed for the PRIME (PReconditioning IMmunostimulatory Evaluation) Phase 1b clinical study of escalating doses of intravenously administered Gemini in patients with Stage 3 and 4 Chronic Kidney Disease (CKD).
By Revelation Biosciences, Inc. · Via Business Wire · July 16, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), announced today that, on July 7, 2025, the Company will implement a 1-for-3 reverse split of its common stock following approval at its Special Meeting of Stockholders held on June 23, 2025. The reverse stock split will be effective as of the morning of July 7, 2025, and the Company’s common stock will trade on a post-split basis at the beginning of trading on the same date under the existing trading symbol “REVB.” The CUSIP number for the common stock following the reverse stock split will be 76135L705.
By Revelation Biosciences, Inc. · Via Business Wire · July 1, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, today announced the closing of its previously announced public offering of 3,640,000 shares of its common stock (or common stock equivalents), together with warrants to purchase up to 14,560,000 shares of its common stock at an offering price to the public of $1.10 per share and associated warrant. The warrants will have an exercise price of $1.10 per share, are exercisable beginning on the effective date of stockholder approval of the issuance of the shares upon exercise of the warrants, and will expire five years following the initial exercise date.
By Revelation Biosciences, Inc. · Via Business Wire · May 29, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, today announced the pricing of a public offering of 3,640,000 shares of its common stock (or common stock equivalents), together with warrants to purchase up to 14,560,000 shares of its common stock at an offering price to the public of $1.10 per share and associated warrant. The warrants will have an exercise price of $1.10 per share, are exercisable beginning on the effective date of stockholder approval of the issuance of the shares upon exercise of the warrants, and will expire five years following the initial exercise date. The closing of the offering is expected to occur on or about May 29, 2025, subject to the satisfaction of customary closing conditions.
By Revelation Biosciences, Inc. · Via Business Wire · May 29, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, announced the retirement of George F. Tidmarsh, MD, PhD from the Company’s Board of Directors.
By Revelation Biosciences, Inc. · Via Business Wire · May 23, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, today reported its three months ended March 31, 2025 financial results.
By Revelation Biosciences, Inc. · Via Business Wire · May 8, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, announced Gemini priming attenuates the inflammatory response in human peripheral blood mononuclear cells (PBMCs) exposed to clinically relevant promoter molecules of inflammation. Revelation anticipates demonstrating the same protective effect in patients treated with Gemini in its Phase 1b clinical study, which will provide evidence of the potential efficacy of Gemini in the Company’s target indications.
By Revelation Biosciences, Inc. · Via Business Wire · March 17, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, announced a new target indication for Gemini for the prevention of infection in severe burn patients requiring hospitalization (the GEM-PBI program). The use of Gemini for the prevention of infection in severe burn patients, as well as the prevention of infection post-surgery (the GEM-PSI program) are a part of the patent family Revelation previously licensed from Vanderbilt University.
By Revelation Biosciences, Inc. · Via Business Wire · April 29, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, announced today that James Rolke Revelation’s Chief Executive Officer, will participate in a fireside chat at the 37th Annual Roth Conference on Tuesday, March 18, 2025 at 10:00 a.m. PT in Dana Point, CA.
By Revelation Biosciences, Inc. · Via Business Wire · March 13, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, today reported its three and twelve months ended December 31, 2024 financial results.
By Revelation Biosciences, Inc. · Via Business Wire · March 6, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, announced dosing of the first patient in the PRIME (PReconditioning IMmunostimulatory Evaluation) Phase 1b clinical study of intravenous single ascending doses of Gemini in patients with Stage 3 and 4 Chronic Kidney Disease (CKD). The US-based, multi-site, placebo-controlled study will enroll up to forty patients in up to five cohorts.
By Revelation Biosciences, Inc. · Via Business Wire · February 26, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation to optimize health, announced today the Nasdaq Stock Market LLC (“Nasdaq”) formally notified the Company that REVB common stock will continue to be listed and traded on Nasdaq, as Revelation is in compliance with all requirements for continued listing.
By Revelation Biosciences, Inc. · Via Business Wire · February 24, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the treatment of disease, announced today that it has started its PRIME (PReconditioning IMmunostimulatory Evaluation) Phase 1b clinical study of escalating doses of intravenously administered Gemini in patients with Stage 3 and 4 Chronic Kidney Disease (CKD). The US based multi-site placebo-controlled study will enroll up to forty patients in five cohorts of single escalating doses.
By Revelation Biosciences, Inc. · Via Business Wire · January 21, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the treatment of disease, wants to remind all shareholders to vote their proxies for the January 17, 2025 special meeting of stockholders. In particular, a vote for Proposal 2, the redomicile of the Company from Delaware to Nevada will save the Company at least $200,000.00 per year in franchise tax fees. All proposals in the proxy are important and the Board of Directors has suggested a vote for all proposals.
By Revelation Biosciences, Inc. · Via Business Wire · January 13, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the treatment of disease, announced today that the Nasdaq Hearings Panel issued a decision letter granting Revelation’s request to continue its listing on The Nasdaq Stock Market, subject to its stock trading at or above $1.00 for at least ten consecutive trading days by February 14, 2025.
By Revelation Biosciences, Inc. · Via Business Wire · January 6, 2025

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the treatment of disease, announced today the entry into a definitive agreement for the immediate exercise of certain outstanding warrants to purchase up to an aggregate of 4,064,040 shares of common stock, issued by the Company on August 22, 2024 (the “Existing Warrants”), at the exercise price of $1.00 per share. The shares of common stock issuable upon exercise of the Existing Warrants are registered pursuant to an effective registration statement on Form S-3 (File No. 333-281909). The closing of the offering is expected to occur on or about December 3, 2024, subject to satisfaction of customary closing conditions.
By Revelation Biosciences, Inc. · Via Business Wire · December 3, 2024

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the treatment of disease, announced today that the United States Food and Drug Administration (FDA) has accepted its investigational new drug (IND) application for Gemini. This game changing milestone allows the Company to initiate its US based Phase 1b clinical study to evaluate the potential of Gemini as a preconditioning treatment in patients with chronic kidney disease (CKD).
By Revelation Biosciences, Inc. · Via Business Wire · December 2, 2024

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage biopharmaceutical company focused on harnessing the power of trained immunity for the prevention and treatment of disease, today announced it has successfully completed the GMP manufacture of Gemini drug product to support anticipated clinical studies in the United States. GMP compliant manufacturing of Gemini is a pivotal requirement for an Investigational New Drug (IND) application with FDA. The Company intends to utilize the current supply of Gemini for the upcoming Phase 1b clinical trial in chronic kidney disease (CKD) patients as well as future Phase 2 studies.
By Revelation Biosciences, Inc. · Via Business Wire · November 12, 2024

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage biopharmaceutical company focused on harnessing the power of trained immunity for the prevention and treatment of disease by developing and commercializing therapeutics that modulate the innate immune system, today reported its three and nine months ended September 30, 2024 financial results.
By Revelation Biosciences, Inc. · Via Business Wire · November 8, 2024
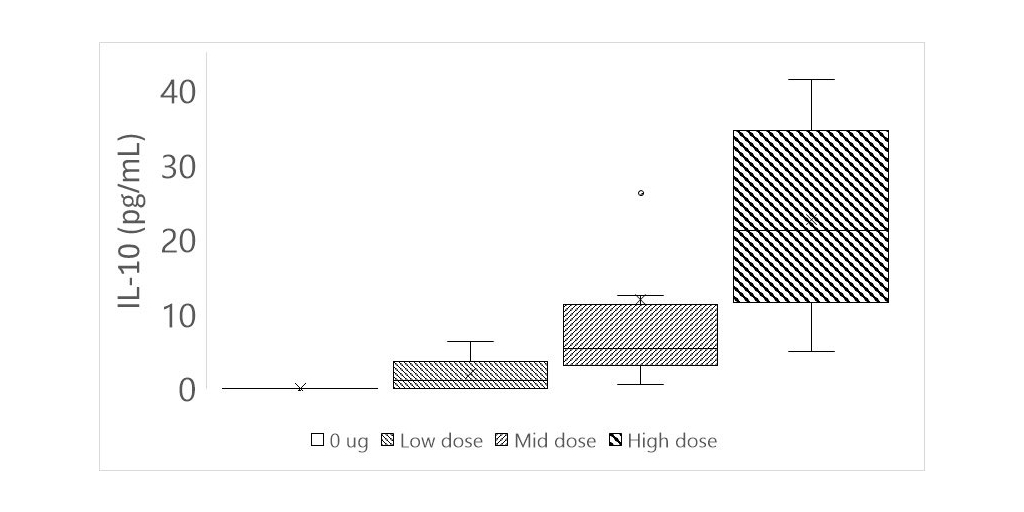
Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the prevention and treatment of disease, today announced statistically significant, dose dependent increases of interleukin-10 (IL-10) in response to Gemini treatment, using a high sensitivity analysis. This additional positive data follows the previously reported positive topline data from the Phase 1 clinical study of Gemini released on June 24, 2024.
By Revelation Biosciences, Inc. · Via Business Wire · September 24, 2024

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage biopharmaceutical company focused on harnessing the power of trained immunity for the prevention and treatment of disease by developing and commercializing therapeutics that modulate the innate immune system, today reported its three and six months ended June 30, 2024 financial results.
By Revelation Biosciences, Inc. · Via Business Wire · August 9, 2024
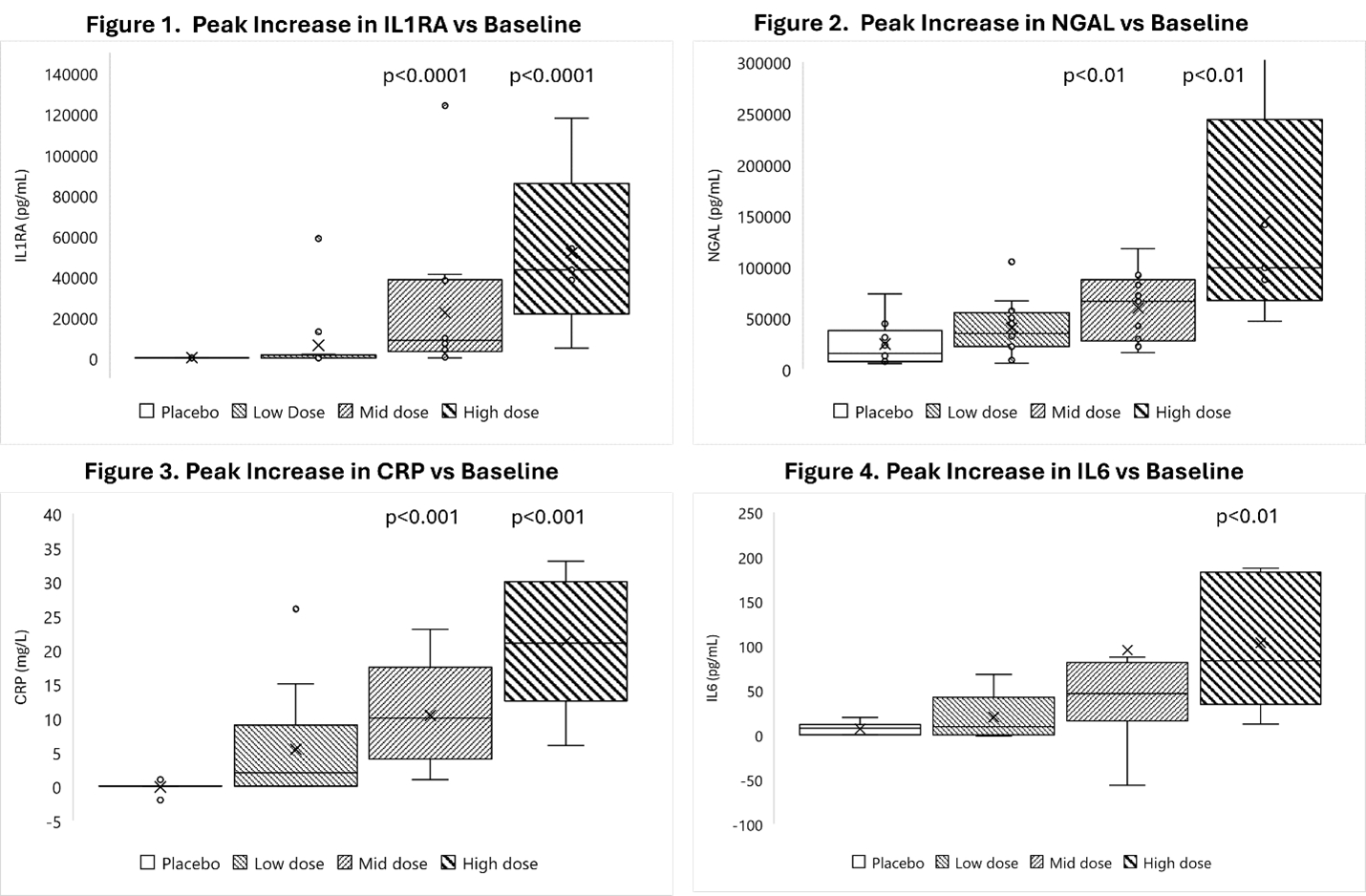
Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the prevention and treatment of disease, today announced safety and biomarker data for its Phase 1 clinical study (RVL-HV02). The primary endpoint to evaluate the safety and tolerability of escalating doses of Gemini was met and a maximum tolerated dose in healthy volunteers was identified. Additionally, statistically significant dose dependent upregulation of key biomarkers demonstrating the immunostimulatory preconditioning effect of Gemini were observed. The study, which enrolled 40 healthy individuals 18 to 55 years of age, was conducted in Australia and evaluated escalating doses (placebo, low, mid and high dose) of intravenously administered Gemini.
By Revelation Biosciences, Inc. · Via Business Wire · June 24, 2024

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the prevention and treatment of disease, announced today that it has completed enrollment and dosing of its first in human Phase 1 clinical study (RVL-HV02). The study, which was conducted in Australia, evaluated escalating doses of intravenously administered Gemini and enrolled 40 healthy individuals 18 to 55 years of age. The forthcoming top-line data will include primary end points of safety and tolerability along with exploratory endpoints including multiple biomarkers of activity to demonstrate stimulation of the innate immune response. If positive, the data from this Phase 1 clinical study will support future program development of Gemini across multiple indications.
By Revelation Biosciences, Inc. · Via Business Wire · June 13, 2024

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the prevention and treatment of disease, today reported its three months ended March 31, 2024 financial results.
By Revelation Biosciences, Inc. · Via Business Wire · May 10, 2024

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on harnessing the power of trained immunity for the prevention and treatment of disease, today reported its three and twelve months ended December 31, 2023 financial results.
By Revelation Biosciences, Inc. · Via Business Wire · March 22, 2024

Revelation Biosciences, Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a life sciences company that is focused on harnessing the power of trained immunity for the prevention and treatment of disease, today reported its three and nine months ended September 30, 2023 financial results.
By Revelation Biosciences, Inc. · Via Business Wire · November 13, 2023

SAN DIEGO, July 26, 2022 (GLOBE NEWSWIRE) -- Revelation Biosciences Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on the development of immunologic-based therapies for the prevention and treatment of disease, today announced the pricing of a public offering of 8,333,334 shares of its common stock, together with warrants to purchase up to 8,333,334 shares of its common stock at an offering price to the public of $0.60 per share and associated warrant. The warrants will have an exercise price of $0.60 per share, are exercisable upon issuance, and will expire five years following the date of issuance. The closing of the offering is expected to occur on or about July 28, 2022, subject to the satisfaction of customary closing conditions.
By Revelation Biosciences, Inc. · Via GlobeNewswire · July 26, 2022

Revelation Biosciences, Inc. (“Revelation”), a clinical-stage life sciences company focused on the development of immunologic‑based therapies for the prevention and treatment of disease, and Petra Acquisition, Inc. (“Petra”) (NASDAQ: PAICU, PAIC, & PAICW), a special purpose acquisition company (“SPAC”), today announced they have entered into a definitive merger agreement for a business combination that will result in Revelation becoming a publicly traded company.
By Revelation Biosciences, Inc. · Via Business Wire · August 30, 2021
