Articles from LEO Pharma

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · January 21, 2026

LEO Pharma, a global leader in medical dermatology, announced today that CEO Christophe Bourdon will deliver a company update at the 44th Annual J.P. Morgan Healthcare Conference in San Francisco on Tuesday, January 13, 2026, at 9:00 AM PST.
By LEO Pharma · Via Business Wire · January 7, 2026

LEO Pharma, a global leader in medical dermatology, announces the appointment of Marika Murto, PhD, as SVP of Global Product Strategy and member of the Global Leadership Team.
By LEO Pharma · Via Business Wire · December 16, 2025

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · December 15, 2025

GLOBAL RELEASE - NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · November 17, 2025

In the first nine months of 2025, LEO Pharma continued its robust revenue growth, with significantly improved profitability and free cash flow. As expected, growth accelerated in the third quarter, with the global rollout of Anzupgo® gaining further momentum after its September launch in the U.S. The 2025 financial outlook is updated to reflect the addition of Spevigo® to the portfolio, reinforcing LEO Pharma’s commitment to advancing innovation and expanding access to care.
By LEO Pharma · Via Business Wire · November 6, 2025

GLOBAL RELEASE - NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · November 5, 2025

LEO Pharma, a leader in medical dermatology, today announces the appointment of Sophie Lamle, D.Phil, as new Executive Vice President (EVP) of Development. Sophie will join the Global Leadership Team and will play an instrumental role in further strengthening LEO Pharma's Search & Development model.
By LEO Pharma · Via Business Wire · November 3, 2025

LEO Pharma A/S, a global leader in medical dermatology, is advancing the understanding of skin disease and its impact on patients’ lives with the presentation of 46 abstracts at the upcoming 2025 Fall Clinical Dermatology Conference taking place from Oct 23 - 26 in Las Vegas, Nevada. The data span multiple forms of skin disease, including chronic hand eczema (CHE), atopic dermatitis (AD), generalized pustular psoriasis (GPP) and pyoderma gangrenosum (PG).
By LEO Pharma · Via Business Wire · October 21, 2025

GLOBAL RELEASE - NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · October 1, 2025

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 19, 2025

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 17, 2025

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 15, 2025

In H1 2025, LEO Pharma delivered robust growth and significantly improved profitability, enabling an increase to the financial outlook for sales growth and adjusted EBITDA margin in 2025 towards the upper-end of previously communicated expectations. In July, the FDA approval of Anzupgo® and partnership with Boehringer Ingelheim for SPEVIGO®, marked major strategic milestones demonstrating LEO Pharma’s commitment to advancing innovation in dermatology.
By LEO Pharma · Via Business Wire · August 18, 2025

GLOBAL RELEASE - NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · July 14, 2025

NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · July 9, 2025

LEO Pharma A/S, a global leader in medical dermatology, today announced the initiation of the phase 2a proof-of-concept DELTA NEXT trial. The trial aims to evaluate the efficacy and safety of delgocitinib cream compared to cream vehicle in the treatment of adults with mild to severe Palmoplantar Pustulosis (PPP).
By LEO Pharma · Via Business Wire · June 3, 2025

In Q1, LEO Pharma continued its robust growth, driven by dermatology, and made significant strategic progress. This included expanding the launch of Anzupgo® to five markets, advancing innovation through the newly formed strategic partnership with Gilead for the STAT6 program, and significantly improving profitability with a return to a positive net profit.
By LEO Pharma · Via Business Wire · May 15, 2025

NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · May 13, 2025

Ninety-five percent (95%) of U.S. dermatology providers surveyed agreed moderate-to-severe chronic hand eczema (CHE) has a strong impact on patients’ work and home life, according to the second phase of the survey sponsored by LEO Pharma Inc., a global leader in medical dermatology, and conducted by Ipsos.
By LEO Pharma · Via Business Wire · April 22, 2025

LEO Pharma A/S, a global leader in medical dermatology, today announced the positive results for the primary endpoint from the double-blind treatment period of the DELTA China trial. DELTA China is a phase 3 clinical trial with Anzupgo® (delgocitinib) 20mg/g cream, a topical pan-Janus kinase (JAK) inhibitor, for the potential treatment of Chinese adults and adolescents (aged 12 and above) with moderate to severe Chronic Hand Eczema (CHE) for whom topical corticosteroids are inadequate or inappropriate.1
By LEO Pharma · Via Business Wire · February 27, 2025

LEO Pharma delivered another year of strong progress in 2024, with both sales growth and adjusted EBITDA margin ahead of the initial outlook for the year, putting the company on track to meet its profitability targets and to achieve its long-term strategic objectives.
By LEO Pharma · Via Business Wire · February 26, 2025

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · February 4, 2025

LEO Pharma, a global leader in medical dermatology, today announced that the Medicines and Healthcare Products Regulatory Agency (MHRA) has granted marketing authorisation for delgocitinib cream for the treatment of adult patients with moderate to severe Chronic Hand Eczema (CHE), for whom topical corticosteroids are inadequate or inappropriate.1
By LEO Pharma · Via Business Wire · December 4, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · November 14, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · October 31, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · October 15, 2024

LEO Pharma today announced its commitment to achieving a net-zero climate target by 2050. It involves developing an extensive decarbonization plan across the company’s operations, aligning with the Paris Agreement and climate science recommendations to limit global warming to 1.5⁰ C.
By LEO Pharma · Via Business Wire · October 11, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 27, 2024

NOT INTENDED FOR DISTRIBUTION IN THE UK
By LEO Pharma · Via Business Wire · September 27, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 26, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 25, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 25, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 23, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 23, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · September 5, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · August 26, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · August 21, 2024

LEO Pharma A/S, a global leader in medical dermatology, today announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion that recommends the approval of Anzupgo® (delgocitinib cream) for the treatment of adult patients with moderate to severe chronic hand eczema (CHE), for whom topical corticosteroids are inadequate or inappropriate.
By LEO Pharma · Via Business Wire · July 26, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · July 19, 2024

GLOBAL RELEASE – NOT INTENDED FOR DISTRIBUTION IN THE UK
By LEO Pharma · Via Business Wire · May 7, 2024
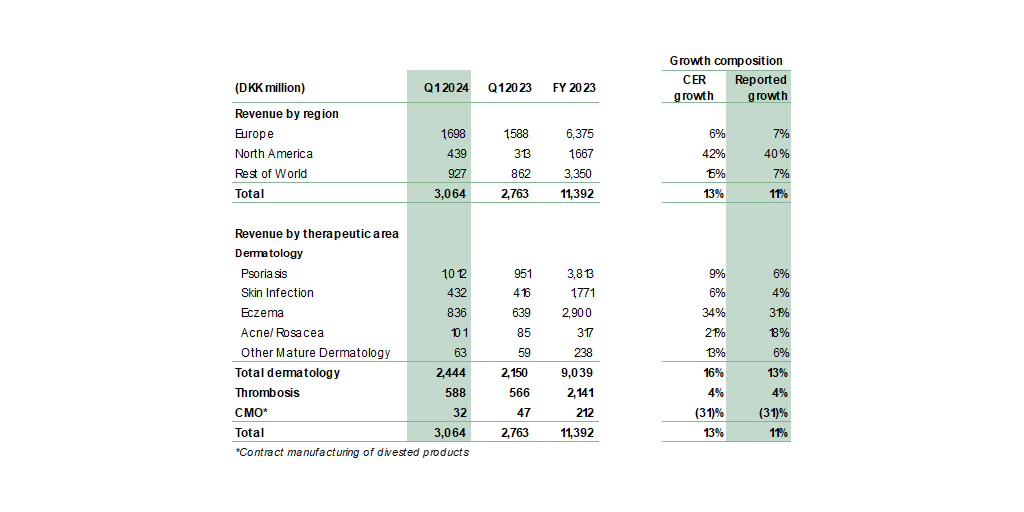
In Q1 2024, LEO Pharma delivered a revenue growth of 13% in constant exchange rates (CER). The dermatology portfolio saw accelerated growth in revenue of 16%. The acquisition of TMB-001 to the treatment of congenital ichthyosis added a late-stage asset to LEO Pharma’s medical dermatology pipeline, and delgocitinib for chronic hand eczema (CHE) is on track for its planned European launch in Q4 2024. Full-year outlook has been revised slightly upwards.
By LEO Pharma · Via Business Wire · May 3, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · March 10, 2024

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · March 6, 2024

LEO Pharma delivers a solid operational performance uplift with double-digit revenue growth and an EBITDA uplift of DKK 2.1 billion, corresponding to a 20%-points margin improvement. LEO Pharma exceeded our profitability guidance and hit the upper range of our revenue growth guidance provided in March 2023.
By LEO Pharma · Via Business Wire · February 29, 2024

LEO Pharma A/S, a global leader in medical dermatology, today announced positive results from the DELTA FORCE trial.
By LEO Pharma · Via Business Wire · January 24, 2024

LEO Pharma today announced that it has finalized the acquisition of the strategic asset TMB-001 as well as certain other assets from Timber Pharmaceuticals following its chapter 11 bankruptcy filing.
By LEO Pharma · Via Business Wire · January 23, 2024

NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · December 15, 2023

LEO Pharma A/S, a global leader in medical dermatology, today announced the positive outcome of the DELTA 3 trial. DELTA 3 is a phase 3, single-arm, open-label extension trial of delgocitinib cream, an investigational topical pan-Janus kinase (JAK)-inhibitor, for the potential treatment of adults with moderate to severe chronic hand eczema (CHE).
By LEO Pharma · Via Business Wire · October 30, 2023

LEO Pharma A/S, a global leader in medical dermatology, today presented results from the DELTA 2 trial at the 32nd European Academy of Dermatology and Venereology (EADV) Congress in Berlin. DELTA 2 is the second of two pivotal phase 3 clinical trials with delgocitinib cream, an investigational topical pan-Janus kinase (JAK)-inhibitor for the potential treatment of adults with moderate to severe chronic hand eczema (CHE).1,4
By LEO Pharma · Via Business Wire · October 13, 2023

LEO Pharma A/S, a global leader in medical dermatology, today presented new data assessing the long-term safety and efficacy data of continuous treatment with Adtralza® (tralokinumab) over four years for moderate-to-severe atopic dermatitis (AD) in adults. Findings were shared during two oral presentations at the 32nd European Academy of Dermatology and Venereology (EADV) Congress in Berlin.1,2
By LEO Pharma · Via Business Wire · October 13, 2023

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · October 11, 2023

LEO Pharma A/S, a global leader in medical dermatology, is today announcing the appointment of Professor Alexander Egeberg, a globally recognized dermatologist and top key opinion leader as the company’s new Vice President, Head of Global Medical Affairs. The announcement happens ahead of the upcoming 32nd European Academy of Dermatology and Venereology (EADV) Congress.
By LEO Pharma · Via Business Wire · October 9, 2023

Today, LEO Pharma announced results for first half 2023 and an update of its capital structure
By LEO Pharma · Via Business Wire · September 12, 2023

Today, LEO Pharma announced the appointment of Nathalie Daste as new Executive Vice President, Global People and Corporate Affairs, and member of its Global Leadership Team, effective October 1, 2023.
By LEO Pharma · Via Business Wire · August 28, 2023

LEO Pharma today announced that it signed an agreement to acquire U.S.-listed Timber Pharmaceuticals, Inc. (NYSE American: TMBR. Upon closing, this transaction will add an attractive late-stage asset to LEO Pharma’s pipeline in medical dermatology. The deal is subject to certain closing conditions including, but not limited to, Timber Pharmaceuticals’ shareholder approval.
By LEO Pharma · Via Business Wire · August 21, 2023

NOT FOR UK USE – NOT INTENDED FOR UK MEDIA
By LEO Pharma · Via Business Wire · August 18, 2023

LEO Pharma A/S, a global leader in medical dermatology, today announced that a Phase 2a trial evaluating the efficacy and safety of investigational agent LEO 138559 in adults with moderate-to-severe atopic dermatitis met its primary endpoint. Results were shared as one of two LEO Pharma late breaker oral presentations at the 2023 American Academy of Dermatology (AAD) Annual Meeting.1 LEO 138559 is an investigational agent and its efficacy and safety are subject to further larger trials.
By LEO Pharma · Via Business Wire · March 18, 2023

LEO Pharma A/S, a global leader in medical dermatology, today presented positive results from the DELTA 1 trial in one of two LEO Pharma late-breaking sessions at the American Academy of Dermatology (AAD) 2023 Annual Meeting.1 DELTA 1 is a pivotal phase 3 clinical trial with delgocitinib cream, an investigational topical pan-Janus kinase (JAK)-inhibitor for the potential treatment of adults with moderate to severe chronic hand eczema (CHE).2 The safety and efficacy of delgocitinib cream is under investigation and has not been evaluated by any health authority.
By LEO Pharma · Via Business Wire · March 18, 2023

LEO Pharma A/S, a global leader in medical dermatology, today presented new clinical data from the ECZTRA 6 and ECZTEND trials of Adbry™ (tralokinumab-ldrm), marketed outside of the U.S. under the tradename Adtralza®, in adolescent patients aged 12 to 17 years with moderate-to-severe atopic dermatitis (AD). The data was presented at the American Academy of Dermatology (AAD) 2023 Annual Meeting. The use of Adbry in adolescent patients aged 12-17 is currently under clinical investigation and the safety and efficacy have not been fully evaluated by the U.S. FDA.
By LEO Pharma · Via Business Wire · March 17, 2023

LEO Pharma today announced a split of its R&D organization into two distinct functions. To lead Research and Early Development, LEO Pharma has appointed Dr. Jacob Pontoppidan Thyssen as new Executive Vice President and Chief Scientific Officer. Jacob is an internationally recognized dermatologist and key opinion leader who will add his clinical experience and strong understanding of patients’ needs into LEO Pharma's drug development.
By LEO Pharma · Via Business Wire · March 14, 2023

LEO Pharma, a global leader in medical dermatology, and ICON plc (NASDAQ: ICLR) today announced a strategic partnership that will enable LEO Pharma to scale clinical trial execution that is patient-centric and cost effective, and which will support the company’s overall ambition of building one of the most effective and efficient clinical portfolio execution organisations in the industry.
By LEO Pharma · Via Business Wire · March 10, 2023

LEO Pharma A/S, a global leader in medical dermatology, today announced positive results of the DELTA 2 trial. DELTA 2 is the second of two pivotal phase 3 clinical trials with delgocitinib cream, an investigational topical pan-Janus kinase (JAK)-inhibitor, for the potential treatment of adults with moderate to severe chronic hand eczema (CHE).
By LEO Pharma · Via Business Wire · February 10, 2023

LEO Pharma A/S, a global leader in medical dermatology, today announced positive results of the DELTA 1 trial. DELTA 1 is the first of two pivotal phase 3 clinical trials with delgocitinib cream, an investigational topical pan-Janus kinase (JAK)-inhibitor, for the potential treatment of adults with moderate to severe chronic hand eczema (CHE).
By LEO Pharma · Via Business Wire · December 6, 2022

LEO Pharma A/S, a global leader in medical dermatology, today launched AD Days Around the World, a global disease awareness campaign that highlights the experiences of people living with atopic dermatitis (AD) – the most common form of eczema.1 In collaboration with patient advocacy organizations in France, Italy, Germany and Spain, the campaign shares real patient stories to educate and inform people living with AD that regardless of nationality or culture, there is hope to triumph over adversity, despite common everyday challenges.
By LEO Pharma · Via Business Wire · October 3, 2022

DKSH Business Unit Healthcare, a leading partner for healthcare companies seeking to grow their business in Asia and beyond, has partnered with LEO Pharma to bring high-quality therapeutic products for dermatology and thrombosis to patients across Asia.
By LEO Pharma · Via Business Wire · September 29, 2022

NOT FOR DISTRIBUTION IN THE UK
By LEO Pharma · Via Business Wire · September 19, 2022

LEO Pharma A/S, a global leader in medical dermatology, today announced new safety data for Adtralza® (tralokinumab) for adult patients with moderate-to-severe atopic dermatitis (AD). Interim results were shared as an oral presentation at the 31st European Academy of Dermatology and Venereology (EADV) Congress.1
By LEO Pharma · Via Business Wire · September 8, 2022

LEO Pharma A/S, a global leader in medical dermatology, today announced new safety data for AdbryTM (tralokinumab-ldrm) for adult patients with moderate-to-severe atopic dermatitis (AD). Interim results were shared as an oral presentation at the 31st European Academy of Dermatology and Venereology (EADV) Congress.1
By LEO Pharma · Via Business Wire · September 8, 2022

LEO Pharma today announced the appointment of Paul Navarre as new member of its Board of Directors.
By LEO Pharma · Via Business Wire · August 31, 2022

LEO Pharma A/S, a global leader in medical dermatology, today announced that American Journal of Clinical Dermatology published 32-week results from a post-hoc analysis of the Phase 3 ECZTRA 3 clinical trial (NCT03363854) in atopic dermatitis (AD). The analysis showed treatment with Adbry™ (tralokinumab-ldrm) plus topical corticosteroids (TCS) as needed demonstrated improvements in extent and severity of AD, sleep interference, and quality of life over 32 weeks in adults with moderate-to-severe AD.1
By LEO Pharma · Via Business Wire · July 20, 2022

As part of LEO Pharma’s transformation towards becoming a global leader in medical dermatology, the company today announced a refocused commercial structure to support building a more simple, agile, and competitive company.
By LEO Pharma · Via Business Wire · June 2, 2022

LEO Pharma is proud to announce the validation of its Scope 1 & 2 and Scope 3 targets by the Science Based Targets initiative (SBTi)1.
By LEO Pharma · Via Business Wire · April 21, 2022

LEO Pharma A/S, a global leader in medical dermatology, today announced it has enrolled the first patient in a Phase 2b dose-ranging clinical trial with an investigational oral histamine receptor 4 (H4R) antagonist (LEO 152020) for the potential treatment of adults with moderate-to-severe atopic dermatitis (AD).
By LEO Pharma · Via Business Wire · December 14, 2021

LEO Pharma A/S, a global leader in medical dermatology, today announced 16-week results of a 52-week monotherapy trial showing tralokinumab significantly improved primary and secondary measurements of efficacy among adolescents (aged 12 to 17) with moderate-to-severe atopic dermatitis.1 The week 16 results from the Phase 3 ECZTRA 6 trial were shared during the 2021 Fall Clinical Dermatology Conference held virtually and with a hybrid option in Las Vegas.
By LEO Pharma · Via Business Wire · October 22, 2021
