Articles from Genentech

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the pivotal Phase III study (FENhance 1) of fenebrutinib in RMS met its primary endpoint, showing clinically meaningful and statistically significant results. The study demonstrated that fenebrutinib, an investigational Bruton’s tyrosine kinase (BTK) inhibitor, markedly reduced the annualized relapse rate (ARR) by 51% compared to teriflunomide over a period of at least 96 weeks of treatment, consistent with FENhance 2 results showing a 59% reduction in ARR. Together, these results equate to approximately one relapse every 17 years. Secondary endpoints in both RMS studies show statistically significant and clinically meaningful reductions in brain lesions. Additionally, all progression endpoints show favorable trends for fenebrutinib.
By Genentech · Via Business Wire · March 2, 2026

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) has approved the combination regimen of Venclexta® (venetoclax) plus acalabrutinib for the treatment of previously untreated adults with chronic lymphocytic leukemia (CLL), based on results from the Phase III AMPLIFY study.
By Genentech · Via Business Wire · February 20, 2026

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) has accepted the company’s New Drug Application for giredestrant, an investigational oral therapy, in combination with everolimus for the treatment of adult patients with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2-negative, ESR1-mutated locally advanced or metastatic breast cancer following recurrence or progression on a prior endocrine-based regimen. The FDA is expected to make a decision on the approval by December 18, 2026. Giredestrant plus everolimus could be the first and only oral selective estrogen receptor degrader (SERD) combination approved in the post-cyclin-dependent kinase (CDK)4/6 inhibitor setting.
By Genentech · Via Business Wire · February 20, 2026

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today that the Phase III MAJESTY study in adults with primary membranous nephropathy met its primary endpoint, showing statistically significant and clinically meaningful results with Gazyva® (obinutuzumab). Results show that significantly more people achieved complete remission at two years (104 weeks) with Gazyva versus tacrolimus. Safety was in line with the well-characterized profile of Gazyva and no new safety signals were identified.
By Genentech · Via Business Wire · February 16, 2026

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today new late-breaking data from the Phase III FENtrepid study showing the investigational Bruton’s tyrosine kinase (BTK) inhibitor fenebrutinib met its primary endpoint of non-inferiority compared to Ocrevus® (ocrelizumab) in reducing disability progression in patients with primary progressive multiple sclerosis (PPMS). Fenebrutinib showed a 12% reduction in the risk of disability progression compared to Ocrevus, the only approved medicine for PPMS, as measured by the time to onset of 12-week composite confirmed disability progression (cCDP12) (hazard ratio [HR] 0.88; 95% confidence interval [CI]: 0.75, 1.03) with curves separating as early as 24 weeks. A consistent treatment effect on cCDP12 was observed across patient subgroups and for the entire treatment duration.
By Genentech · Via Business Wire · February 7, 2026

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive topline results from CT388-103, a Phase II clinical trial of CT-388, an investigational dual GLP-1/GIP receptor agonist being developed for the treatment of obesity. The study found that once-weekly subcutaneous injections of CT-388 (titrated up to 24 mg) resulted in significant and clinically meaningful placebo-adjusted weight loss of 22.5% (efficacy estimand) without reaching a weight loss plateau at 48 weeks. A clear dose-response relationship on the weight loss was observed. For the treatment-regimen estimand, the placebo-adjusted weight loss achieved with CT-388 was 18.3% (p-value < 0.001). At week 48 for the 24 mg dose, 95.7% of CT-388 treated participants achieved a weight loss of ≥5%, 87% achieved ≥10%, 47.8% achieved ≥20%, and 26.1% achieved ≥30%. 73% of participants who were pre-diabetic at baseline and treated with CT-388 at 24 mg achieved normal blood glucose levels at week 48 compared to 7.5% in the placebo group.
By Genentech · Via Business Wire · January 27, 2026

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today announced an expansion of its initial investment in a new Holly Springs, North Carolina manufacturing facility. The increased investment will more than double the total commitment for the company’s first-ever East Coast manufacturing facility to approximately $2 billion. The expansion builds on the company’s May 2025 investment announcement, as well as the project’s August 2025 groundbreaking, and reflects Genentech’s continued confidence in the region’s community, workforce, and long-term growth potential.
By Genentech · Via Business Wire · January 20, 2026

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) has approved CD20xCD3 bispecific Lunsumio VELO™ (mosunetuzumab-axgb), as a subcutaneous (SC) formulation, for the treatment of adult patients with relapsed or refractory (R/R) follicular lymphoma (FL) after two or more lines of systemic therapy, based on results from the Phase I/II GO29781 study. Based on the study results, Lunsumio VELO is approved under accelerated approval. Full approval for this regimen may be contingent on verification and confirmation of benefit in a confirmatory trial.
By Genentech · Via Business Wire · December 22, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced an agreement with the U.S. government that lowers costs for state Medicaid programs, encourages other wealthy countries to reward innovation, and increases opportunities for direct patient access.
By Genentech · Via Business Wire · December 19, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive data from the Phase III lidERA Breast Cancer study evaluating investigational giredestrant as an adjuvant endocrine treatment for people with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2-negative, early-stage breast cancer. At the pre-specified interim analysis, adjuvant giredestrant significantly reduced the risk of invasive disease recurrence or death by 30% (invasive disease-free survival [iDFS]) compared with standard-of-care endocrine therapy (SoC ET) (hazard ratio [HR]=0.70, 95% confidence interval [CI] 0.57-0.87, p=0.0014). The lidERA results are being presented at the 2025 San Antonio Breast Cancer Symposium and are included in the official press program.
By Genentech · Via Business Wire · December 10, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today new data highlighting the potential of Lunsumio® (mosunetuzumab-axgb) in earlier treatment lines for people living with different types of lymphoma, presented at the 67th American Society of Hematology Annual Meeting and Exposition, December 6-9, 2025 in Orlando, Florida.
By Genentech · Via Business Wire · December 8, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive Phase III results from the lidERA Breast Cancer study evaluating investigational giredestrant as an adjuvant endocrine treatment for people with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, early-stage breast cancer. The study met its primary endpoint at a pre-planned interim analysis, showing a statistically significant and clinically meaningful improvement in invasive disease-free survival with giredestrant versus standard-of-care endocrine therapy. lidERA is the first Phase III trial of a selective estrogen receptor degrader (SERD) to demonstrate a significant benefit in the adjuvant setting. The majority of breast cancer cases are diagnosed at an early stage.
By Genentech · Via Business Wire · November 18, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the first Phase III (FENhance 2) of two pivotal, similarly-designed Phase III studies (FENhance 1 and 2) in patients with relapsing multiple sclerosis (RMS) met its primary endpoint. Fenebrutinib, an investigational Bruton’s tyrosine kinase (BTK) inhibitor, significantly reduced the annualized relapse rate (ARR) compared to teriflunomide over a period of at least 96 weeks of treatment.
By Genentech · Via Business Wire · November 10, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that it will showcase 46 abstracts, including 12 oral presentations, from its industry-leading hematology portfolio at the 67th American Society of Hematology (ASH) Annual Meeting and Exposition, held December 6-9, 2025 in Orlando, Florida.
By Genentech · Via Business Wire · November 3, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today statistically significant and clinically meaningful results from the Phase III ALLEGORY study of Gazyva® (obinutuzumab) in adults with systemic lupus erythematosus (SLE) on standard therapy. The study met its primary endpoint showing a higher percentage of people achieved a minimum four-point improvement in SLE Responder Index 4 (SRI-4) at one year (52 weeks) with Gazyva versus standard therapy. SRI is a tool that assesses changes in disease severity, symptoms and physical condition to indicate whether treatment is effective at controlling disease activity. All key secondary endpoints were also met. No new safety signals were identified, and safety was in line with the well-characterized profile of Gazyva.
By Genentech · Via Business Wire · November 3, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today statistically significant and clinically meaningful results from the Phase III INShore study of Gazyva® (obinutuzumab) in children and young adults (aged ≥ 2-25 years) with idiopathic nephrotic syndrome (INS). The study met its primary endpoint, with more people achieving sustained complete remission at one year (week 52) with Gazyva compared with mycophenolate mofetil (MMF). Sustained complete remission was defined by the absence of relapses during the study together with a low amount of protein in the urine (protein to creatine of 0.2 or less) at week 52. Certain important key secondary endpoints were also met. No new safety signals were identified and safety was in line with the well-characterized profile of Gazyva in adults.
By Genentech · Via Business Wire · October 28, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive results from the Phase III IMvigor011 study evaluating Tecentriq® (atezolizumab) as an adjuvant treatment for people with muscle-invasive bladder cancer (MIBC) who are at risk of recurrence after surgery (cystectomy) and have detectable circulating tumor DNA (ctDNA). In this ctDNA-guided setting, Tecentriq reduced the risk of death (overall survival, OS) by 41% and the risk of disease recurrence or death (disease-free survival, DFS) by 36%, both compared with placebo. This ctDNA-guided approach, using Natera’s Signatera™ ctDNA Molecular Residual Disease (MRD) test, spared people at low risk of recurrence from unnecessary treatment and side effects. The safety profile was consistent with previous studies of Tecentriq.
By Genentech · Via Business Wire · October 20, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) has approved Gazyva® (obinutuzumab) for the treatment of adult patients with active lupus nephritis (LN) who are receiving standard therapy, as well as a shorter 90-minute infusion time after the first infusion, for eligible patients. Following four initial doses in the first year, Gazyva can be administered twice yearly, offering an effective and potentially more convenient treatment option than traditional targeted therapies.
By Genentech · Via Business Wire · October 20, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive results from the Phase III evERA Breast Cancer study. Data showed giredestrant in combination with everolimus significantly reduced the risk of disease progression or death (progression-free survival; PFS) by 44% and 62% in the intention-to-treat (ITT) and ESR1-mutated populations, respectively, compared with standard-of-care endocrine therapy plus everolimus. The evERA study is evaluating the investigational giredestrant combination in people with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer previously treated with cyclin-dependent kinase (CDK) 4/6 inhibitor and endocrine therapy. This is the first positive head-to-head Phase III trial investigating a selective estrogen receptor degrader-containing regimen versus a standard-of-care combination. The results are being presented in an oral session at the European Society for Medical Oncology Congress 2025. Data will be shared with health authorities, with the aim of bringing this potential treatment option to people as soon as possible.
By Genentech · Via Business Wire · October 18, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today results from two Phase III studies evaluating the efficacy and safety of two doses of investigational vamikibart (0.25 and 1 mg) compared with a sham procedure that mimics intravitreal (IVT) injections in people with uveitic macular edema (UME). UME is characterized by the buildup of fluid in the macula due to uveitis, an inflammatory condition of the eye, that can result in vision loss. Across both studies, the primary and secondary endpoint data support the potential for rapid improvements in vision and reductions in macular thickness (swelling in the back of the eye due to retinal fluid) with vamikibart treatment. The data were presented at the American Academy of Ophthalmology annual meeting (AAO 2025) in Orlando, FL.
By Genentech · Via Business Wire · October 17, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today announced the company’s first Direct-to-Patient (DTP) program. This program, which supports President Trump’s goal to make medicines more affordable for American patients, applies to Xofluza® (baloxavir marboxil), a single-dose oral antiviral medication used to treat the flu in patients ages five and up as well as those who have been exposed to the flu. This initiative will help reduce costs for people who don’t have insurance, have limited coverage, or whose health plans don’t cover Xofluza.
By Genentech · Via Business Wire · October 16, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that it will present more than 30 abstracts across more than 10 cancer types at the European Society for Medical Oncology (ESMO) Congress 2025, held October 17-21, 2025 in Berlin, Germany. The data underscore Genentech’s commitment to deliver transformative medicines for some of the most challenging cancer types, including breast cancers, lung cancers, gastrointestinal and genitourinary cancers.
By Genentech · Via Business Wire · October 13, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) has approved Tecentriq® (atezolizumab) and Tecentriq Hybreza® (atezolizumab and hyaluronidase-tqjs) in combination with lurbinectedin (Zepzelca®) for the maintenance treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC) whose disease has not progressed after first-line induction therapy with Tecentriq or Tecentriq Hybreza, carboplatin and etoposide (CE). This approval marks the first and only combination therapy for the first-line maintenance treatment of ES-SCLC, a highly aggressive disease for which treatment options have been limited. The U.S. National Comprehensive Cancer Network® Clinical Practice Guidelines in Oncology (NCCN Guidelines®)* have been updated to include the regimen as a category 2A and preferred option for maintenance treatment of people with ES-SCLC, following induction therapy with Tecentriq and CE.
By Genentech · Via Business Wire · October 2, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), presents new data for Ocrevus® (ocrelizumab) and the investigational Bruton’s tyrosine kinase (BTK) inhibitor fenebrutinib at the 41st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) in Barcelona, Spain (September 24-26).
By Genentech · Via Business Wire · September 24, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive results from the Phase III evERA study evaluating investigational giredestrant in combination with everolimus in people with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer previously treated with a cyclin-dependent kinase (CDK) 4/6 inhibitor and endocrine therapy. The study met both co-primary endpoints, demonstrating a statistically significant and clinically meaningful improvement in progression-free survival (PFS) in both the intention-to-treat and ESR1-mutated populations, compared with standard-of-care endocrine therapy plus everolimus. Overall survival (OS) data were immature, but a clear positive trend was observed. Follow-up continues to the next OS analysis. The giredestrant combination was well tolerated. Adverse events were consistent with the known safety profiles of the individual study treatments, and no new safety signals were observed. This is the first positive head-to-head Phase III trial investigating an all-oral selective estrogen receptor degrader-containing regimen versus a standard of care combination.
By Genentech · Via Business Wire · September 22, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today new data from the AVONELLE-X and SALWEEN studies of Vabysmo® (faricimab-svoa), presented at the 25th Euretina Congress in Paris, France. Data from the open-label AVONELLE-X study reinforce the efficacy, safety and durability of Vabysmo over four years in wet age-related macular degeneration (AMD), a leading cause of vision loss. In the single-arm SALWEEN study, Vabysmo showed clinically meaningful vision gains and retinal drying over one year in polypoidal choroidal vasculopathy (PCV), a vision-threatening subtype of wet AMD that is especially common in Asia.
By Genentech · Via Business Wire · September 5, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) and Alnylam (Nasdaq: ALNY) today announced the decision to initiate a Phase III cardiovascular outcomes trial (CVOT) to evaluate the ability of zilebesiran, an RNAi therapeutic, to reduce the risk of major adverse cardiovascular events in patients with uncontrolled hypertension. This decision was informed by the comprehensive KARDIA Phase II program, including KARDIA-1, KARDIA-2 and the most recent KARDIA-3 study evaluating the efficacy and safety of zilebesiran in patients with uncontrolled hypertension and high cardiovascular (CV) risk, on two to four standard of care antihypertensives. In particular, KARDIA-3 aimed to define the patient population to be investigated in the Phase III CV outcomes trial.
By Genentech · Via Business Wire · August 30, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today broke ground on its newest U.S. manufacturing site in Holly Springs, North Carolina. This significant development marks the establishment of Genentech’s first manufacturing facility on the East Coast. The 700,000-square-foot facility is strategically designed to support production of the company’s future portfolio of metabolic medicines, including next-generation treatments for obesity. The event was attended by federal, state and local officials, including U.S. Rep. Deborah Ross, North Carolina Gov. Josh Stein, State Sec. of Commerce Lee Lilley, State Sens. Sydney Batch and Lisa Grafstein, State Rep. Erin Paré, Wake County Commissioner Cheryl Stallings and Holly Springs Mayor Sean Mayefskie.
By Genentech · Via Business Wire · August 25, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today new, five-year efficacy, safety and durability data from the Phase III Portal study, a long-term extension of the Phase III Archway study, of Susvimo® (ranibizumab injection) for the treatment of people with wet AMD. Results show that Susvimo’s immediate and predictable durability was sustained over five years, with approximately 95% of people receiving treatment every six months requiring no supplemental treatment before each refill. The data were presented at the American Society of Retina Specialists (ASRS) 2025 Annual Meeting in Long Beach, California.
By Genentech · Via Business Wire · August 1, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today that new data from its Alzheimer’s development portfolio is being presented at the Alzheimer’s Association International Conference (AAIC) in Toronto, Canada (July 27-30). These data exemplify the comprehensive approach Roche is taking in addressing Alzheimer’s across the entire patient journey.
By Genentech · Via Business Wire · July 28, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today topline results from the pivotal Phase IIb ALIENTO (n=1,301) and the Phase III ARNASA (n=1,375) trials investigating astegolimab compared to placebo, on top of standard of care maintenance therapy in people with moderate to very severe chronic obstructive pulmonary disease (COPD). The studies included a broad population: both former and current smokers, regardless of blood eosinophil count, who have a history of frequent exacerbations.
By Genentech · Via Business Wire · July 21, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) issued a Complete Response Letter (CRL) for Genentech’s supplemental Biologics License Application (sBLA) for Columvi® (glofitamab-gxbm) in combination with gemcitabine and oxaliplatin (GemOx) for the treatment of people with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are not candidates for autologous stem cell transplant.
By Genentech · Via Business Wire · July 18, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), presented today results from the Phase III SUNMO [NCT05171647] study showing Lunsumio® (mosunetuzumab-axgb) administered subcutaneously in combination with Polivy® (polatuzumab vedotin-piiq) demonstrated a clinically meaningful and statistically significant improvement in its primary endpoints of progression-free survival (PFS) and objective response rate (ORR) compared to Rituxan® (rituximab), gemcitabine and oxaliplatin (R-GemOx), in people with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) who are not eligible for transplant. Primary analysis data were featured at the 18th International Conference on Malignant Lymphoma as a late-breaking oral presentation.
By Genentech · Via Business Wire · June 20, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), and AbbVie announced today the outcome from the Phase III VERONA study (NCT04401748) investigating Venclexta® (venetoclax) plus azacitidine for patients with previously untreated higher-risk myelodysplastic syndromes (MDS). The study did not meet the primary endpoint of overall survival at the final analysis. The safety profile of the Venclexta combination was consistent with the known risk of the individual study medicines and no unexpected safety signals were observed. Full data will be presented at an upcoming medical meeting in 2025.
By Genentech · Via Business Wire · June 16, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today its decision to proceed with Phase III development of prasinezumab, an investigational anti-alpha-synuclein antibody, in early-stage Parkinson’s disease. This decision is informed by data from the Phase IIb PADOVA study and ongoing open-label extensions (OLEs) of PADOVA and Phase II PASADENA studies.
By Genentech · Via Business Wire · June 16, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today positive results from the Phase III IMforte study of Tecentriq® (atezolizumab) in combination with lurbinectedin (Zepzelca®) as a first-line maintenance treatment for people with extensive-stage small cell lung cancer (ES-SCLC), following induction therapy with carboplatin, etoposide and Tecentriq. The data showed that this combination reduced the risk of disease progression or death by 46% and the risk of death by 27%, compared to Tecentriq maintenance therapy alone. Safety was consistent with the known safety profiles of Tecentriq and lurbinectedin. These data are being presented in an oral session at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting and simultaneously published in The Lancet.
By Genentech · Via Business Wire · June 2, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive final results from the overall survival (OS) analysis of the Phase III INAVO120 study. These data showed ItovebiTM (inavolisib), in combination with palbociclib (Ibrance®) and fulvestrant, reduced the risk of death by more than 30% compared with palbociclib and fulvestrant alone. This represents a statistically significant and clinically meaningful improvement in overall survival for people with PIK3CA-mutated, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, endocrine-resistant, locally advanced or metastatic breast cancer. The results are being presented in an oral session at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting and simultaneously published in the New England Journal of Medicine (NEJM).
By Genentech · Via Business Wire · May 31, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today new, 96-week data for fenebrutinib demonstrating that patients with relapsing multiple sclerosis (RMS) maintained no disability progression and low levels of disease activity for up to two years. The latest results for this investigational Bruton’s tyrosine kinase (BTK) inhibitor from the Phase II FENopta open-label extension (OLE) study were presented at the Consortium of Multiple Sclerosis Centers (CMSC) Annual Meeting in Phoenix, Arizona.
By Genentech · Via Business Wire · May 30, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today two-year follow-up data from the Phase III STARGLO study. After a median follow-up of 24.7 months, data showed a 40% improvement in overall survival (OS) for patients treated with Columvi® (glofitamab-gxbm) in combination with gemcitabine and oxaliplatin (GemOx) and median OS was not reached, compared to 13.5 months for Rituxan® (rituximab) plus GemOx (R-GemOx). These updated data continue to demonstrate the statistically significant and clinically meaningful survival benefit of this off-the-shelf, fixed-duration Columvi combination for people with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who have received at least one prior line of therapy and are not candidates for autologous stem cell transplant (ASCT). Data will be presented in an oral session at the 61st American Society of Clinical Oncology (ASCO), May 30 – June 3, 2025.
By Genentech · Via Business Wire · May 23, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today that the U.S. Food and Drug Administration (FDA) has approved Susvimo® (ranibizumab injection) 100 mg/mL for the treatment of diabetic retinopathy (DR), a potentially blinding condition that affects almost 10 million people in the U.S. and more than 100 million people globally. It is the first and only FDA-approved continuous delivery treatment shown to maintain vision in people with DR with just one refill every nine months. Susvimo is now available to U.S. retina specialists and their patients with DR who have previously responded to at least two anti-vascular endothelial growth factor (VEGF) injections.
By Genentech · Via Business Wire · May 22, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that a U.S. Food and Drug Administration (FDA) Oncologic Drugs Advisory Committee (ODAC) discussed the supplemental Biologics License Application (sBLA) for Columvi® (glofitamab-gxbm) in combination with gemcitabine and oxaliplatin (GemOx) for the treatment of people with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are not candidates for autologous stem cell transplant (ASCT).
By Genentech · Via Business Wire · May 20, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), the Breast International Group (BIG), Institut Jules Bordet Clinical Trials Support Unit and Frontier Science Foundation, announced today statistically significant final overall survival (OS) results from the Phase III APHINITY study in people with human epidermal growth factor receptor 2 (HER2)-positive early-stage breast cancer. After ten years, the risk of death was reduced by 17% for people treated with Perjeta® (pertuzumab), Herceptin® (trastuzumab) and chemotherapy (the Perjeta-based regimen) for a year as post-surgery (adjuvant) treatment, compared with individuals who received Herceptin, chemotherapy, and placebo.
By Genentech · Via Business Wire · May 13, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today plans to invest more than $700 million in a new 700,000 square foot state-of-the-art drug manufacturing facility in Holly Springs, NC.
By Genentech · Via Business Wire · May 12, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today that the New England Journal of Medicine (NEJM) has published a detailed analysis of the Phase III CENTERSTONE trial of Xofluza® (baloxavir marboxil). The trial met its primary endpoint, showing a single, oral dose of Xofluza taken by people infected with influenza reduced the odds of untreated household members contracting the virus by 32%. For the key secondary endpoint of influenza virus transmission resulting in symptoms, Xofluza showed a clinically meaningful reduction although statistical significance was not reached. Xofluza was well tolerated, with no new safety signals identified.
By Genentech · Via Business Wire · April 25, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that new data were presented at the AD/PD 2025 International Conference on Alzheimer’s and Parkinson’s Diseases in Vienna, Austria. Highlights included presentations from the ongoing trontinemab Phase Ib/IIa Brainshuttle™ AD study demonstrating dose-dependent rapid amyloid depletion from the brain and the potential of the Elecsys® pTau181 plasma test to rule out amyloid pathology. A Phase III program for trontinemab will be initiated later this year.
By Genentech · Via Business Wire · April 3, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the Phase III MUSETTE trial comparing a high dose of Ocrevus® (ocrelizumab) intravenous (IV) infusion to the currently approved Ocrevus IV 600 mg dose in people with relapsing multiple sclerosis (RMS) did not meet its primary endpoint in showing additional benefit in slowing disability progression, as measured by a composite disability endpoint over a period of at least 120 weeks of treatment. The rates of disability progression were low and consistent with rates observed in the previous pivotal studies of Ocrevus IV 600 mg. In addition, in several predefined analyses on disease activity, Ocrevus IV 600 mg showed clinically meaningful results with the lowest annualized relapse rate (ARR) observed during the double-blind period of a Phase III study in RMS. The MUSETTE data further support the efficacy and safety profile of the currently approved Ocrevus IV 600 mg dose for RMS.
By Genentech · Via Business Wire · April 2, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today that the U.S. Food and Drug Administration (FDA) has accepted the company’s supplemental Biologics License Application (sBLA) for Gazyva® (obinutuzumab) for the treatment of lupus nephritis. The filing acceptance is based on positive results from the Phase III REGENCY study, which showed improved complete renal response (CRR) with Gazyva plus standard therapy compared with standard therapy alone. The FDA is expected to make a decision on approval by October 2025.
By Genentech · Via Business Wire · March 5, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today announced that the U.S. Food and Drug Administration (FDA) has approved TNKase® (tenecteplase), a thrombolytic or clot-dissolving agent, for the treatment of acute ischemic stroke (AIS) in adults. This approval of TNKase marks Genentech’s second approval for stroke, reinforcing the company's long-standing dedication to advancing stroke care as the developer of the only two FDA-approved medicines for AIS, TNKase and Activase® (alteplase).
By Genentech · Via Business Wire · March 3, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today new positive data from Stage 2 and Stage 3 of the National Institutes of Health (NIH)-sponsored Phase III OUtMATCH study, which provide further evidence supporting the role of Xolair® (omalizumab) for the treatment of one or more food allergies. Stage 2 of the OUtMATCH study showed Xolair was more effective with fewer side effects than multi-allergen oral immunotherapy (OIT) in the first-ever head-to-head trial comparing the two treatment approaches. OIT involves ingesting the food allergen, initially with a very small amount and gradually increasing the amount. These findings were largely driven by the high rates of adverse events (AEs) leading to study discontinuation in the OIT-treated group.
By Genentech · Via Business Wire · March 2, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) has approved a New Drug Application (NDA) for an Evrysdi® (risdiplam) tablet for people living with spinal muscular atrophy (SMA). Evrysdi is the only non-invasive disease-modifying treatment for SMA. The 5 mg Evrysdi tablet can either be swallowed whole or dispersed in water.
By Genentech · Via Business Wire · February 12, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that a detailed analysis of its Phase III REGENCY trial of Gazyva® (obinutuzumab) in people with active lupus nephritis (LN) was published in the New England Journal of Medicine. The trial demonstrated a statistically significant and clinically meaningful improvement in the primary endpoint of complete renal response (CRR), showing that 46.4% of people treated with Gazyva plus standard therapy (mycophenolate mofetil and glucocorticoids) achieved CRR at 76 weeks compared with 33.1% of people treated with standard therapy alone (adjusted difference 13.4%, 95% CI, 2.0%-24.8%; p=0.0232). This was accompanied by clinically meaningful improvements in complement levels and reductions in anti-dsDNA, markers of disease activity and inflammation.
By Genentech · Via Business Wire · February 7, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today that the U.S. Food and Drug Administration (FDA) has approved Susvimo® (ranibizumab injection) 100 mg/mL for the treatment of diabetic macular edema (DME), a leading cause of vision loss in adults with diabetes, affecting more than 29 million adults worldwide. Susvimo is the first and only FDA-approved treatment shown to maintain vision in people with DME with fewer treatments than standard-of-care eye injections. Susvimo is now available to U.S. retina specialists and their patients with DME.
By Genentech · Via Business Wire · February 4, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive topline results from the overall survival (OS) analysis of the Phase III INAVO120 study investigating ItovebiTM (inavolisib) in combination with palbociclib (Ibrance®) and fulvestrant for people with PIK3CA-mutated, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, endocrine-resistant, locally advanced or metastatic breast cancer. The study met its key secondary endpoint, showing a statistically significant and clinically meaningful OS benefit with the Itovebi-based regimen compared with palbociclib and fulvestrant alone.
By Genentech · Via Business Wire · January 28, 2025

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today results from the Phase IIb PADOVA study investigating prasinezumab in 586 people with early-stage Parkinson’s disease, treated for a minimum of 18 months while on stable symptomatic treatment. Prasinezumab showed potential clinical efficacy in the primary endpoint of time to confirmed motor progression with a HR=0.84 [0.69-1.01] and p=0.0657, missing statistical significance. In a pre-specified analysis, the effect of prasinezumab was more pronounced in the population treated with levodopa (75% of participants), HR=0.79 [0.63-0.99]. Consistent positive trends across multiple secondary and exploratory endpoints were also observed. Prasinezumab continues to be well tolerated and no new safety signals were observed in the study.
By Genentech · Via Business Wire · December 19, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that new and updated data from its industry-leading CD20xCD3 T-cell-engaging bispecific antibody program were presented at the 66th American Society of Hematology (ASH) Annual Meeting & Exposition, December 7-10, 2024. With more than 20 bispecific antibody abstracts accepted for presentation, data showcase the benefits of fixed-duration Columvi® (glofitamab-gxbm) and Lunsumio® (mosunetuzumab-axgb) across different types of aggressive and indolent lymphomas. This research supports Genentech’s efforts to continue innovating for patients by advancing treatment standards at earlier stages of disease while exploring additional forms of administration that could further improve the patient experience.
By Genentech · Via Business Wire · December 9, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today data from a five-year follow-up of the pivotal Phase III POLARIX study evaluating Polivy® (polatuzumab vedotin-piiq) in combination with Rituxan® (rituximab), cyclophosphamide, doxorubicin and prednisone (R-CHP) in people with untreated diffuse large B-cell lymphoma (DLBCL). Data were presented in an oral session at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition, December 7-10, 2024 in San Diego, California. This latest analysis conducted after a median follow-up of 60.9 months, includes descriptive data on primary and secondary endpoints, as well as safety results.
By Genentech · Via Business Wire · December 8, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) has accepted the company’s supplemental Biologics License Application (sBLA) for Columvi® (glofitamab-gxbm) in combination with gemcitabine and oxaliplatin (GemOx) for the treatment of people with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who have received at least one prior line of therapy and are not candidates for autologous stem cell transplant. The FDA is expected to make a decision on approval by July 20, 2025.
By Genentech · Via Business Wire · December 5, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), reports an update on the Phase III SKYSCRAPER-01 study, evaluating tiragolumab combined with Tecentriq® (atezolizumab) compared to Tecentriq alone for patients with PD-L1-high, locally advanced or metastatic non-small cell lung cancer (NSCLC).
By Genentech · Via Business Wire · November 26, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today that it will present more than 40 abstracts across nine blood disorders at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition, held December 7-10, 2024 in San Diego, California. The data underscore Genentech’s commitment to advance patient outcomes in lymphoma with long-term follow-up of its approved medicines Polivy® (polatuzumab vedotin-piiq), Lunsumio® (mosunetuzumab-axgb) and Columvi® (glofitamab-gxbm) as well as new investigational combination data.
By Genentech · Via Business Wire · November 5, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that a detailed analysis of the positive Phase III INAVO120 results, evaluating ItovebiTM (inavolisib) in combination with palbociclib (Ibrance®) and fulvestrant was published in the New England Journal of Medicine. The U.S. Food and Drug Administration (FDA) recently approved Itovebi in combination with palbociclib and fulvestrant, for the treatment of adults with endocrine-resistant, PIK3CA-mutated, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer, as detected by an FDA-approved test, following recurrence on or after completing adjuvant endocrine therapy. Data from INAVO120 are also being used for filing submissions to other global health authorities, including the European Medicines Agency.
By Genentech · Via Business Wire · October 30, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive topline one-year results from the open-label, single-arm Phase IV ELEVATUM study evaluating Vabysmo® (faricimab-svoa) for the treatment of diabetic macular edema (DME) in people from racial and ethnic groups that are often underrepresented in clinical trials.
By Genentech · Via Business Wire · October 18, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), presented positive 2-year data from the ongoing RAINBOWFISH study at the 29th World Muscle Society (WMS) Congress, October 8-12, 2024, assessing the efficacy and safety of Evrysdi® (risdiplam) in children with SMA who were treated pre-symptomatically as infants before 6 weeks of age (n=23). The study found the majority of children achieved key motor milestones, were able to swallow and feed orally, and demonstrated cognitive skills typical of children without SMA, with none requiring permanent ventilation.
By Genentech · Via Business Wire · October 14, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) approved Itovebi™ (inavolisib), in combination with palbociclib (Ibrance®) and fulvestrant, for the treatment of adults with endocrine-resistant, PIK3CA-mutated, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer, as detected by an FDA-approved test, following recurrence on or after completing adjuvant endocrine therapy. The PIK3CA mutation is found in approximately 40% of HR-positive metastatic breast cancers.
By Genentech · Via Business Wire · October 10, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive topline results from the Phase III REGENCY study of Gazyva® (obinutuzumab) in people with active lupus nephritis. In the study, a higher proportion of people treated with Gazyva plus standard therapy (mycophenolate mofetil and glucocorticoids) achieved a complete renal response (CRR) at 76 weeks compared to those treated with standard therapy alone. Safety was in line with the well-characterized profile of Gazyva. No new safety signals were identified.
By Genentech · Via Business Wire · September 26, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive topline results of the Phase III CENTERSTONE study of Xofluza® (baloxavir marboxil), an antiviral, showing a reduction in the transmission of influenza viruses. The study met its primary endpoint, demonstrating that a single, oral dose of Xofluza taken by people infected with influenza significantly reduced the likelihood of others in their household contracting the virus. Xofluza was well tolerated with no new safety signals identified.
By Genentech · Via Business Wire · September 19, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the United States Food and Drug Administration (U.S. FDA) has approved Ocrevus Zunovo™ (ocrelizumab & hyaluronidase-ocsq) for the treatment of relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS). Ocrevus Zunovo is the first and only twice-a-year, healthcare professional (HCP)-administered approximately 10-minute subcutaneous (SC) injection approved for both these forms of multiple sclerosis, giving people living with MS more treatment options.
By Genentech · Via Business Wire · September 13, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. Food and Drug Administration (FDA) has approved Tecentriq Hybreza™ (atezolizumab and hyaluronidase-tqjs), the first and only PD-(L)1 inhibitor for subcutaneous (SC), under the skin injection for patients in the United States. Tecentriq Hybreza can be injected subcutaneously over approximately seven minutes, compared with 30-60 minutes for standard IV infusion of Tecentriq (atezolizumab). It will be available for all IV indications of Tecentriq approved for adults in the U.S., including certain types of lung, liver, skin and soft tissue cancer.
By Genentech · Via Business Wire · September 12, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today two-year data from the Phase III Pagoda and Pavilion studies evaluating Susvimo® (ranibizumab injection) 100 mg/mL for the treatment of diabetic macular edema (DME) and diabetic retinopathy (DR), respectively, the two leading causes of vision loss in adults with diabetes. Susvimo is the first and only refillable eye implant that provides continuous delivery of a customized formulation of ranibizumab via the Port Delivery Platform. The results build on the primary one-year analyses of the Pagoda and Pavilion studies, with Susvimo demonstrating sustained efficacy over two years and safety consistent with the known safety profile for Susvimo in people with DME and DR. Detailed results were presented at the American Society of Retina Specialists (ASRS) 2024 Annual Meeting in Stockholm, Sweden.
By Genentech · Via Business Wire · July 18, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today new, four-year data from the RHONE-X extension study. The study met all primary endpoints, showing that Vabysmo® (faricimab-svoa) was well tolerated in people with diabetic macular edema (DME) who received treatment for up to four years. Exploratory results from the long-term study showed that Vabysmo continued to preserve vision, dry retinal fluid that can impair sight and allow extended time between treatments in people with DME. These data were presented in a late-breaking oral presentation at the American Society of Retina Specialists (ASRS) 2024 Annual Meeting in Stockholm, Sweden.
By Genentech · Via Business Wire · July 17, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today positive topline results from two arms of an ongoing multi-part Phase I clinical trial for CT-996, an investigational, once-daily, oral small molecule GLP-1 receptor agonist being developed for the treatment of both type 2 diabetes and obesity.1 The data showed that treatment with CT-996 in participants with obesity and without type 2 diabetes resulted in a clinically meaningful placebo-adjusted mean weight loss of -6.1% within four weeks (p <0.001).1 The full study data will be presented at an upcoming medical meeting.
By Genentech · Via Business Wire · July 17, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today the reintroduction of Susvimo® (ranibizumab injection) 100 mg/mL for intravitreal use via ocular implant for the treatment of people in the United States (U.S.) with wet, or neovascular, age-related macular degeneration (AMD), following the end of a voluntary recall. The U.S. Food and Drug Administration (FDA) has approved a post-approval supplement to the Biologics License Application for Susvimo, reflecting component-level updates made to the ocular implant and refill needle. Genentech will work to make Susvimo available in the U.S. to retina specialists and their patients with wet AMD in the coming weeks.
By Genentech · Via Business Wire · July 8, 2024
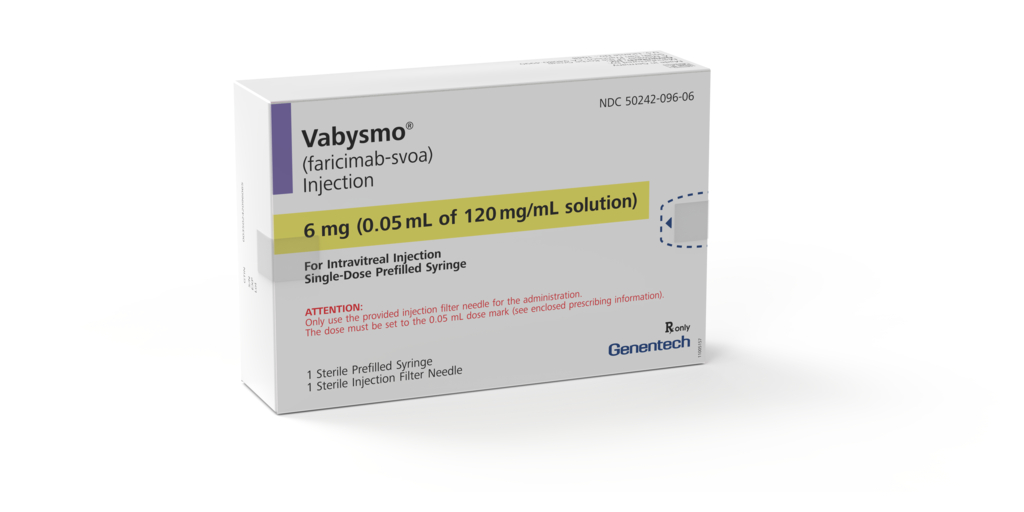
Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY) announced today the U.S. Food and Drug Administration (FDA) has approved the Vabysmo® (faricimab-svoa) 6.0 mg single-dose prefilled syringe (PFS) for use in the treatment of wet, or neovascular, age-related macular degeneration (AMD), diabetic macular edema (DME) and macular edema following retinal vein occlusion (RVO). Together, these three conditions affect close to three million people in the U.S. The Vabysmo PFS will become available to U.S. retina specialists and their patients in the coming months.
By Genentech · Via Business Wire · July 5, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the Phase II/III SKYSCRAPER-06 study, evaluating tiragolumab plus Tecentriq® (atezolizumab) and chemotherapy versus pembrolizumab and chemotherapy as an initial (first-line) treatment for people with previously untreated, locally advanced unresectable or metastatic non-squamous non-small cell lung cancer (nSq NSCLC), did not meet its primary endpoints of progression-free survival (PFS) at its primary analysis with a hazard ratio (HR) of 1.27 [95% CI: 1.02,1.57] and overall survival (OS) at its first interim analysis with a HR of 1.33 [95% CI: 1.02, 1.73], which was immature. The combination of tiragolumab plus Tecentriq and chemotherapy showed reduced efficacy in both PFS and OS compared to the comparator arm in the intent-to-treat population, which includes Phase II and Phase III cohorts. The overall safety profile remains consistent with the safety profile previously observed for the combination of tiragolumab plus Tecentriq and chemotherapy, and no new or unexpected findings were identified. Based on these results, patients and investigators will be unblinded and we intend to halt the study. A communication will be sent to the investigators and results will be shared with health authorities and subsequently presented at an upcoming medical meeting.
By Genentech · Via Business Wire · July 4, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today statistically significant and clinically meaningful results from its Phase III STARGLO study of Columvi® (glofitamab-gxbm) in combination with gemcitabine and oxaliplatin (GemOx) versus Rituxan® (rituximab) in combination with GemOx (R-GemOx) for people with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who have received at least one prior line of therapy and are not candidates for autologous stem cell transplant, or who have received two or more prior lines of therapy. Data were featured in the congress Press Briefing and presented today in the Plenary Abstracts Session at the European Hematology Association (EHA) 2024 Congress as a late-breaking oral presentation.
By Genentech · Via Business Wire · June 15, 2024

Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today new 5-year data confirming the sustained efficacy and safety profile of Evrysdi® (risdiplam) in children with Type 1 spinal muscular atrophy (SMA) from the open-label extension of the pivotal FIREFISH study. By the end of year 5, 91% of children treated with Evrysdi were alive, 81% were alive without permanent ventilation and the majority were able to sit without support for at least 30 seconds (59%). At the end of year 5, seven children were able to stand, three with support, four unaided and six could walk with support. Without disease modifying treatment, natural history studies indicate that children with Type 1 SMA would not only never be able to reach such milestones, but also not typically live past the age of two. The data were presented at the Cure SMA Research & Clinical Care Meeting, June 5 - 7, 2024.
By Genentech · Via Business Wire · June 7, 2024
