Articles from GenSight Biologics

Regulatory News:
By GenSight Biologics · Via Business Wire · December 2, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · October 30, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · October 7, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · September 29, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · July 17, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · July 8, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · June 26, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · June 12, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · May 13, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · April 23, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · April 7, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · March 19, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · March 17, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · February 12, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · January 23, 2025

Regulatory News:
By GenSight Biologics · Via Business Wire · October 17, 2024

Regulatory News:
By GenSight Biologics · Via Business Wire · July 11, 2024

Regulatory News:
By GenSight Biologics · Via Business Wire · June 4, 2024

Regulatory News:
By GenSight Biologics · Via Business Wire · June 3, 2024

Regulatory News:
By GenSight Biologics · Via Business Wire · May 7, 2024

Regulatory News:
By GenSight Biologics · Via Business Wire · April 18, 2024

Regulatory News:
By GenSight Biologics · Via Business Wire · April 4, 2024
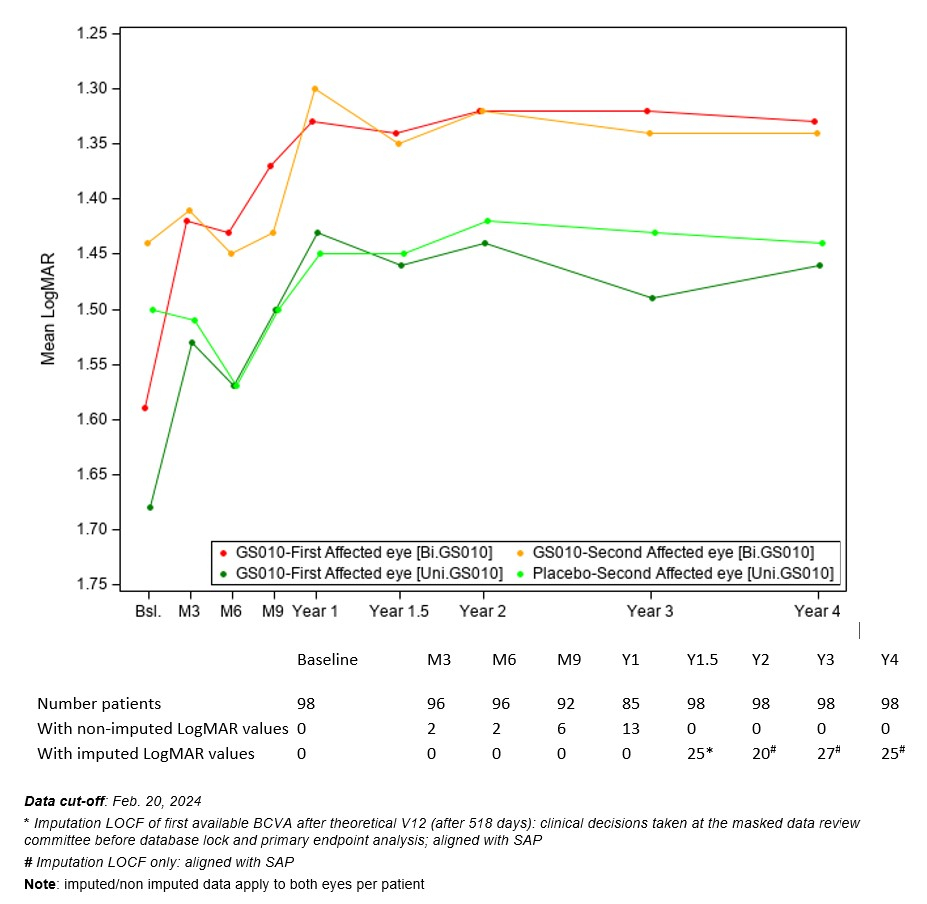
Regulatory News:
By GenSight Biologics · Via Business Wire · March 20, 2024

Regulatory News:
By GenSight Biologics · Via Business Wire · March 4, 2024

Regulatory News:
By GenSight Biologics · Via Business Wire · February 13, 2023

Regulatory News:
By GenSight Biologics · Via Business Wire · December 15, 2022
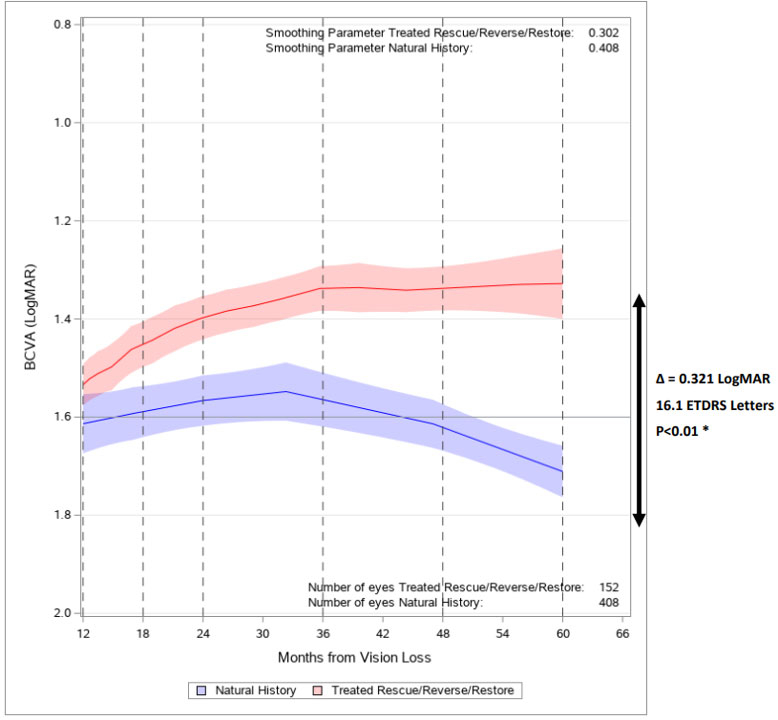
Regulatory News:
By GenSight Biologics · Via Business Wire · July 20, 2022

Regulatory News:
By GenSight Biologics · Via Business Wire · December 1, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · November 22, 2021

GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported a second case of a patient with late-stage retinitis pigmentosa (RP) who partially recovered her visual function after treatment with GS030 optogenetic therapy.
By GenSight Biologics · Via Business Wire · November 17, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · November 12, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · October 12, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · September 15, 2021

GenSight Biologics (Paris:SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced that its gene therapy LUMEVOQ® has been granted Promising Innovative Medicine (PIM) designation by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of vision loss due to Leber Hereditary Optic Neuropathy (LHON) caused by a confirmed G11778A mutation in the ND4 mitochondrial gene.
By GenSight Biologics · Via Business Wire · September 6, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · August 31, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · July 5, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · June 30, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · June 10, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · June 1, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · May 31, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · May 19, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · May 17, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · May 3, 2021

Regulatory News:
By GenSight Biologics · Via Business Wire · April 20, 2021
