Articles from CatalYm

CatalYm today announced that the first patient has been dosed in the randomized Phase 2b GDFATHER‑NSCLC‑02 (GDF‑15 Antibody‑MediaTed‑Human‑Effector‑T‑Cell Relocation) trial (NCT07246863). The trial evaluates the company’s lead anti-GDF-15 antibody visugromab as a second-line treatment in patients with metastatic non-squamous non-small cell lung cancer (nsq NSCLC) who have progressed following initial systemic treatment including an approved immune checkpoint inhibitor.
By CatalYm · Via Business Wire · December 2, 2025

CatalYm, a world-leader in neutralizing GDF-15 in cancer and cachexia, today presented compelling primary results from the Phase 2 GDFATHER-NEO trial in an oral late-breaking session at the European Society of Medical Oncology (ESMO) Congress 2025. The data demonstrated that Growth Differentiation Factor-15 (GDF-15) blockade by visugromab enhanced the efficacy of PD-1 inhibition by nivolumab as a neoadjuvant therapy in muscle-invasive bladder cancer (MIBC), with a similar safety profile compared to nivolumab plus placebo. Visugromab is a humanized, monoclonal antibody designed to neutralize the tumor-derived cytokine GDF-15 which plays a central role in immune suppression and anti-PD-(L)1 treatment resistance.
By CatalYm · Via Business Wire · October 17, 2025

CatalYm today announced that the first patient has been dosed in the randomized Phase 2b GDFATHER-NSCLC-01 trial (NCT07098988). The trial investigates the efficacy and safety of the company’s anti-GDF-15 antibody visugromab, in combination with standard-of-care chemoimmunotherapy, compared to placebo plus chemoimmunotherapy, as a first-line treatment for patients with newly diagnosed metastatic non-squamous non-small cell lung cancer (NSQ mNSCLC).
By CatalYm · Via Business Wire · September 30, 2025

CatalYm, a world-leader in neutralizing GDF-15 in cancer and cachexia, today announced two upcoming oral presentations highlighting the company’s lead-candidate visugromab at the European Society of Medical Oncology (ESMO) Congress, being held October 17th-21st, 2025, in Berlin, Germany. Visugromab is a humanized, monoclonal antibody designed to neutralize the tumor-derived cytokine Growth Differentiation Factor-15 (GDF-15) that plays a central role in immune suppression and anti-PD(L)-1 treatment resistance.
By CatalYm · Via Business Wire · September 22, 2025

CatalYm today announced the appointment of Scott Clarke as Chief Executive Officer. Mr. Clarke brings over two decades of executive leadership experience in driving company growth, developing products, and shaping and executing transactions in the biopharmaceutical industry. He takes the helm as CatalYm enters a new stage of corporate and clinical development, including the initiation of a broad Phase 2b program for its lead candidate, visugromab, in non-squamous non-small-cell lung cancer and additional tumor indications. Based in the US, he will oversee both EU and US operations.
By CatalYm · Via Business Wire · January 9, 2025

CatalYm today announced that data of the first-in-human Phase 1/2a study of its lead drug candidate visugromab were published in Nature. The paper titled “Neutralizing GDF-15 can overcome anti-PD-(L)1 resistance in solid tumors” emphasizes the significant potential of visugromab to induce unprecedented cancer remission depth and durability across multiple solid tumor indications as well as in combination with nivolumab in late- to last-line solid tumors. Visugromab is a humanized, monoclonal antibody that counteracts GDF-15, a critical immunosuppressant used by tumor cells to survive.
By CatalYm · Via Business Wire · December 11, 2024

CatalYm today announced new findings from its ongoing Phase 1/2a study as well as preclinical research, highlighting the therapeutic potential of its lead drug candidate, visugromab, to mitigate cancer cachexia by neutralizing the Growth Differentiation Factor 15 (GDF-15). Cachexia, a condition affecting a significant number of advanced cancer patients, leads to severe weight loss, muscle wasting, and reduced quality of life, often limiting the ability to tolerate cancer therapies. The data were presented at the 17th International Conference on Sarcopenia, Cachexia & Wasting Disorders (SCWD).
By CatalYm · Via Business Wire · December 10, 2024

CatalYm today announced the completion of a $150 million Series D financing. The oversubscribed round was led by new investors, Canaan Partners and Bioqube Ventures, and joined by Forbion's Growth Opportunities Fund (“Forbion Growth”), Omega Funds and Gilde Healthcare. Existing investors Jeito Capital, Brandon Capital Partners, Novartis Venture Fund and Vesalius Biocapital III also participated in the round. The proceeds will fund the expansion of the company’s broad Phase 2b development of visugromab into randomized Phase 2b studies in select checkpoint naïve frontline and second-line treatment settings. Visugromab has already demonstrated outstanding anti-tumor activity in combination with checkpoint inhibitor treatment.
By CatalYm · Via Business Wire · July 16, 2024

CatalYm today announced that positive new follow-up results from its ongoing “GDFATHER” Phase 1/2a trial (GDF-15 Antibody-mediaTed Human Effector Cell Relocation Phase 1/2a) will be featured in an oral presentation at the American Society of Clinical Oncology (ASCO) Annual Meeting 2024 in Chicago.
By CatalYm · Via Business Wire · May 23, 2024

CatalYm today announced the appointment of Dr. Roy Baynes as an independent member to its Board of Directors, as well as the appointment of Dr. Petros Grivas, Dr. Roy Herbst, and Prof. Andrea Necchi to the Scientific Advisory Board. With their combined industry insights, drug development expertise and scientific know-how, the new appointees will greatly support CatalYm with the late-stage clinical evaluation of the company’s lead candidate visugromab.
By CatalYm · Via Business Wire · January 8, 2024

CatalYm announced that maturing Phase 2a results from its ongoing GDFATHER-2 trial (GDF-15 Antibody-mediaTed Human Effector Cell Relocation Phase 2) (NCT04725474) were presented today in an oral presentation at the European Society for Medical Oncology (ESMO) Immuno-Oncology Congress 2023 in Geneva, Switzerland. The data highlights that treatment with a combination of CatalYm’s lead candidate visugromab and nivolumab achieves compelling anti-tumoral activity in (as per strict criteria) anti-PD-1/PD-L1 relapsed/refractory non-small cell lung cancer (NSCLC) and urothelial cancer (UC) patients while retaining an excellent safety and tolerability profile. Visugromab is a monoclonal antibody designed to neutralize the tumor-produced Growth Differentiation Factor-15 (GDF-15), a central mediator of immune resistance to cancer therapies. The presentation by International Coordinating Investigator Prof. Ignacio Melero, MD, PhD, Co-Director of Immunology and Immunotherapy (CIMA) at the Universidad de Navarra, Pamplona/Spain, expands the clinical data for visugromab significantly and highlights the benefits that neutralizing GDF-15 can provide for patients with metastatic solid tumors.
By CatalYm · Via Business Wire · December 6, 2023

CatalYm today announced that the first patient has been dosed in the Phase 2 clinical trial, GDFather-NEO (NCT06059547), evaluating its lead anti-GDF-15 antibody candidate, visugromab, in combination with neoadjuvant immunotherapy (nivolumab, aPD-1 inhibitor) in muscle invasive bladder cancer (MIBC). The exploratory study expands visugromab’s clinical Phase 2 evaluation to a therapeutic front-line setting, building on the positive results from the ongoing GDFather-2 (NCT04725474) trial in last-line metastatic patients with a range of advanced, treatment-resistant solid tumors.
By CatalYm · Via Business Wire · October 26, 2023

CatalYm today announced new preclinical data expanding the mechanistic understanding of how GDF-15 plays a key role in cancer therapy resistance. The results will be presented in a poster session at the European Society for Medical Oncology (ESMO) Congress 2023 in Madrid, Spain on Sunday, October 22nd by CatalYm’s CSO, Dr. Christine Schuberth-Wagner. The data are the first to show that GDF-15 has an inhibitory effect on the activation of M1 macrophages. These specific myeloid lineage immune cells are central to the initiation of immune responses, including the secretion of pro-inflammatory cytokines and chemokines, presentation of antigens as well as direct antitumor cytotoxicity.
By CatalYm · Via Business Wire · October 15, 2023
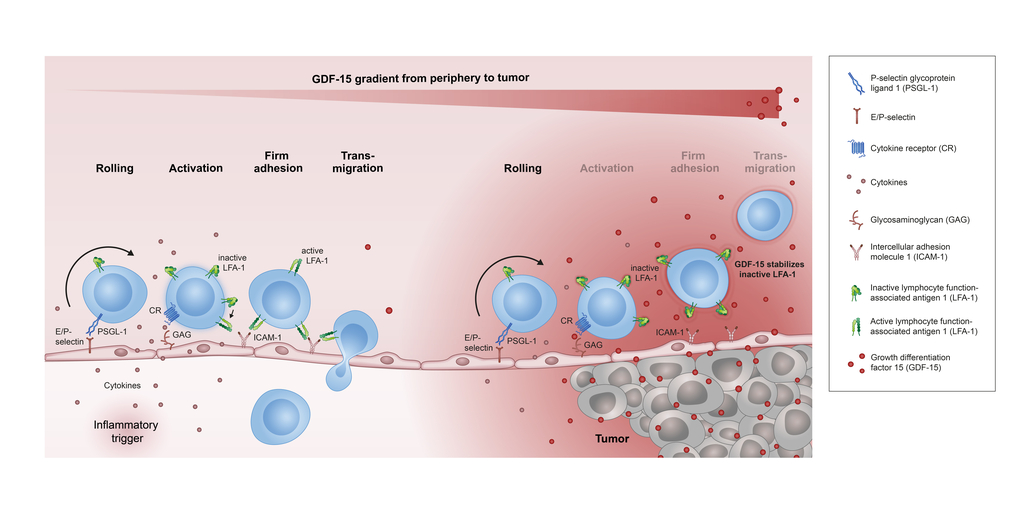
CatalYm today announced the publication of preclinical data in Nature Communications under the title “Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment”. The study reveals a central role of GDF-15 in the resistance of tumors to current immunotherapy. These findings further highlight the therapeutic significance of CatalYm’s proprietary anti-GDF-15 antibody candidate, visugromab, currently in advanced Phase 2 clinical studies.
By CatalYm · Via Business Wire · July 20, 2023

CatalYm today announced first Phase 2a data from its ongoing GDFather-2 trial (GDF-15 Antibody-mediaTed Human Effector Cell Relocation Phase 2) (NCT04725474) at the American Society of Clinical Oncology (ASCO) Annual Meeting 2023 in Chicago, Illinois. The early data presented during today’s oral “Developmental Therapeutics-Immunotherapy” session revealed lasting and confirmed responses in several solid tumor types investigated following treatment with visugromab and the anti-PD-1 inhibitor nivolumab. In addition, the combination continues to demonstrate a good safety and tolerability profile across all cohorts. CatalYm’s lead candidate, visugromab, is a humanized, monoclonal antibody designed to neutralize the tumor-produced Growth Differentiation Factor-15 (GDF-15), a central regulator of tumor resistance development.
By CatalYm · Via Business Wire · June 5, 2023

CatalYm today announced that the first results from its ongoing Phase 2a trial “GDFATHER-2” (GDF-15 Antibody-mediaTed Human Effector Cell Relocation Phase 2) will be featured in an oral presentation at the American Society of Clinical Oncology (ASCO) Annual Meeting 2023 in Chicago. The trial is evaluating CatalYm’s lead GDF-15 neutralizing antibody visugromab in combination with immune checkpoint inhibitor nivolumab in last-line, anti-PD-1/PD-L1 relapsed/refractory patients. Growth and differentiation factor 15 (GDF-15) is recognized as a negative regulator of antitumoral T cell activity preventing T cell recruitment to the tumor microenvironment as well as potently suppressing an adaptive immune response by additional mechanisms recently identified. The ASCO Annual Meeting will be held in Chicago, Illinois, from June 2 to 6, 2023.
By CatalYm · Via Business Wire · May 25, 2023

CatalYm today announced three major expansions of its ongoing Phase 2 visugromab clinical development program. Based on initial positive patient responses, the company is advancing two potential lead indication cohorts into the second stage of the Simon-2-stage design ahead of schedule. Additionally, CatalYm is expanding its visugromab Phase 2 development program to include a new confirmatory cohort exploring the response-predictive biomarkers identified in the Phase 1.
By CatalYm · Via Business Wire · December 14, 2022

CatalYm today announced the close of a EUR 50 million (USD 49 million) Series C financing round. The oversubscribed round was co-led by new investors, Brandon Capital and Jeito Capital with participation from existing investors Forbion, Novartis Venture Fund, Vesalius Biocapital III, Bayern Kapital, BioGeneration Ventures and Coparion. The financing will support the continued, promising clinical development of its lead candidate, visugromab, a humanized monoclonal antibody engineered to neutralize the tumor-produced Growth Differentiation Factor-15 (GDF-15). GDF-15 acts as a key regulator of immune cell activation and as an inhibitor of immune cell infiltration into the tumor tissue.
By CatalYm · Via Business Wire · November 22, 2022

CatalYm today announced that it has received Investigational New Drug (IND) clearance from the United States Food and Drug Administration (FDA) to expand its ongoing Phase 2 clinical program to include clinical trial centers in the U.S. The ongoing GDFATHER-2 program (GDF-15-neutralizing antibody-mediated human effector cell relocation) is evaluating the company’s lead candidate, Visugromab in combination with an anti-PD1 antibody in patients with advanced solid tumors that are relapsed/refractory to prior anti-PD1/-PD-L1 treatment. Visugromab is a monoclonal antibody that neutralizes GDF-15, a key immunosuppressor, which has been shown to prevent T cell migration into tumors, enabling cancerous cells to evade the immune system.
By CatalYm · Via Business Wire · September 26, 2022

CatalYm today announced that the mature results from its Phase 1, first-in-human trial “GDFATHER-1” (GDF-15 antibody-mediated human effector cell relocation) will be presented in an oral presentation at the European Society for Medical Oncology (ESMO) Congress 2022. The trial evaluated CatalYm’s lead GDF-15 neutralizing antibody visugromab (previously known as CTL-002), in combination with immune checkpoint inhibitor nivolumab in last-line, anti-PD-1/PD-L1 relapsed/refractory patients. Growth and differentiation factor 15 (GDF-15) is recognized as a negative regulator of antitumoral T cell activity preventing T cell recruitment to the tumor microenvironment as well as potently suppressing an adaptive immune response by additional mechanisms recently identified. The ESMO congress will be held in Paris, France, from September 9 to 13, 2022.
By CatalYm · Via Business Wire · September 4, 2022

CatalYm today announced the presentation of expanded clinical and new preclinical data for its lead candidate CTL-002, an antibody targeting the novel cancer target GDF-15, at the 36th Society for Immunotherapy of Cancer (SITC) Annual Meeting. The results will be presented in two poster presentations on November 13, 2021 and will include data from the ongoing first-in-human trial “GDFather” (GDF-15 Antibody-mediated Effector cell Relocation) as well as preclinical data characterizing the potential of GDF-15 as a therapeutic target for the treatment of multiple solid tumor indications. The conference is taking place, both in person (Washington, D.C.) and virtually, from November 10 - 14, 2021.
By CatalYm · Via Business Wire · November 12, 2021
